Input interpretation

carbon | specific heat at STP
Result
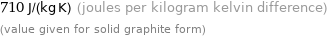
710 J/(kg K) (joules per kilogram kelvin difference) (value given for solid graphite form)
Unit conversions
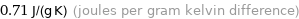
0.71 J/(g K) (joules per gram kelvin difference)
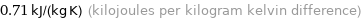
0.71 kJ/(kg K) (kilojoules per kilogram kelvin difference)
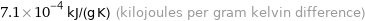
7.1×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

710 J/(kg °C) (joules per kilogram degree Celsius difference)
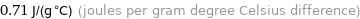
0.71 J/(g °C) (joules per gram degree Celsius difference)
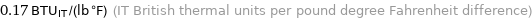
0.17 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons
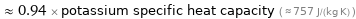
≈ 0.94 × potassium specific heat capacity ( ≈ 757 J/(kg K) )
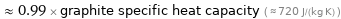
≈ 0.99 × graphite specific heat capacity ( ≈ 720 J/(kg K) )
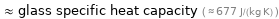
≈ glass specific heat capacity ( ≈ 677 J/(kg K) )
Thermodynamic properties
![phase at STP | solid | melting point | [5, 6]-fullerene-C70 | 280 °C | buckminsterfullerene | 280 °C | activated charcoal | 3550 °C | graphite | 3675 °C boiling point | activated charcoal | 4027 °C molar heat of fusion | graphite | 117.4 kJ/mol molar heat of vaporization | 715 kJ/mol (rank: 3rd) | specific heat at STP | 710 J/(kg K) (rank: 16th) | (properties at standard conditions)](../image_source/d1ea48744d5bc7201d7a634f60dbdb29.png)
phase at STP | solid | melting point | [5, 6]-fullerene-C70 | 280 °C | buckminsterfullerene | 280 °C | activated charcoal | 3550 °C | graphite | 3675 °C boiling point | activated charcoal | 4027 °C molar heat of fusion | graphite | 117.4 kJ/mol molar heat of vaporization | 715 kJ/mol (rank: 3rd) | specific heat at STP | 710 J/(kg K) (rank: 16th) | (properties at standard conditions)