Input interpretation
diazane | tin(II) iodide
Chemical names and formulas
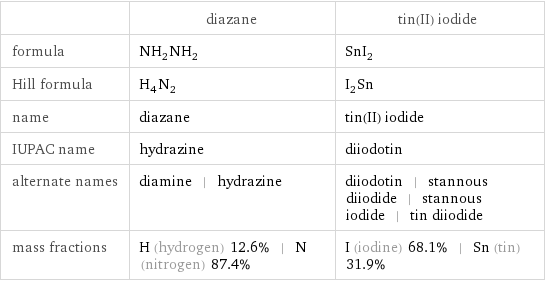
| diazane | tin(II) iodide formula | NH_2NH_2 | SnI_2 Hill formula | H_4N_2 | I_2Sn name | diazane | tin(II) iodide IUPAC name | hydrazine | diiodotin alternate names | diamine | hydrazine | diiodotin | stannous diiodide | stannous iodide | tin diiodide mass fractions | H (hydrogen) 12.6% | N (nitrogen) 87.4% | I (iodine) 68.1% | Sn (tin) 31.9%
Structure diagrams
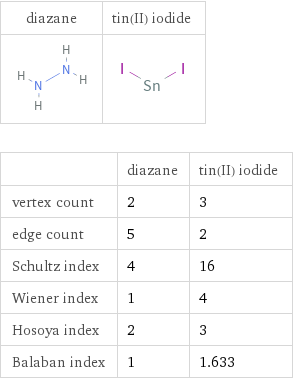
| diazane | tin(II) iodide vertex count | 2 | 3 edge count | 5 | 2 Schultz index | 4 | 16 Wiener index | 1 | 4 Hosoya index | 2 | 3 Balaban index | 1 | 1.633
3D structure
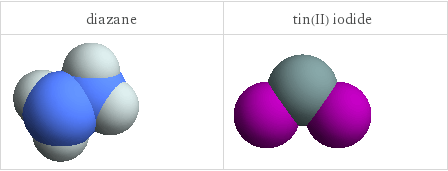
3D structure
Basic properties
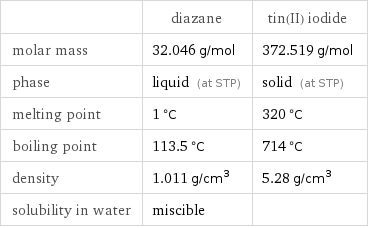
| diazane | tin(II) iodide molar mass | 32.046 g/mol | 372.519 g/mol phase | liquid (at STP) | solid (at STP) melting point | 1 °C | 320 °C boiling point | 113.5 °C | 714 °C density | 1.011 g/cm^3 | 5.28 g/cm^3 solubility in water | miscible |
Units
