Input interpretation
helium (chemical element) | chlorine (chemical element) | neon (chemical element)
Periodic table location
Periodic table location
Images
Images
Basic elemental properties
![| helium | chlorine | neon atomic symbol | He | Cl | Ne atomic number | 2 | 17 | 10 short electronic configuration | 1s^2 | [Ne]3s^23p^5 | [He]2s^22p^6 Aufbau diagram | 1s | 3p 3s | 2p 2s block | s | p | p group | 18 | 17 | 18 period | 1 | 3 | 2 atomic mass | 4.002602 u | 35.45 u | 20.1797 u half-life | (stable) | (stable) | (stable)](../image_source/8b267b893576b78c7b79568c451adbf5.png)
| helium | chlorine | neon atomic symbol | He | Cl | Ne atomic number | 2 | 17 | 10 short electronic configuration | 1s^2 | [Ne]3s^23p^5 | [He]2s^22p^6 Aufbau diagram | 1s | 3p 3s | 2p 2s block | s | p | p group | 18 | 17 | 18 period | 1 | 3 | 2 atomic mass | 4.002602 u | 35.45 u | 20.1797 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
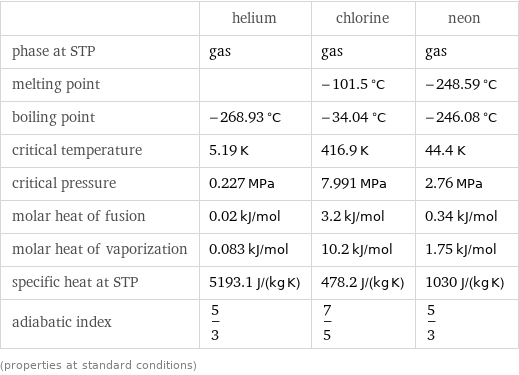
| helium | chlorine | neon phase at STP | gas | gas | gas melting point | | -101.5 °C | -248.59 °C boiling point | -268.93 °C | -34.04 °C | -246.08 °C critical temperature | 5.19 K | 416.9 K | 44.4 K critical pressure | 0.227 MPa | 7.991 MPa | 2.76 MPa molar heat of fusion | 0.02 kJ/mol | 3.2 kJ/mol | 0.34 kJ/mol molar heat of vaporization | 0.083 kJ/mol | 10.2 kJ/mol | 1.75 kJ/mol specific heat at STP | 5193.1 J/(kg K) | 478.2 J/(kg K) | 1030 J/(kg K) adiabatic index | 5/3 | 7/5 | 5/3 (properties at standard conditions)
Material properties
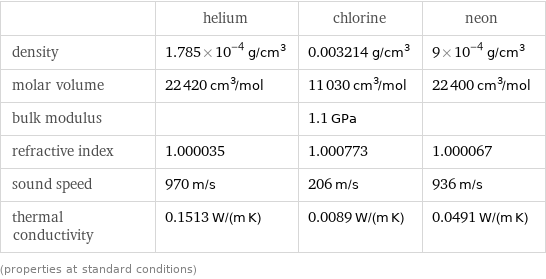
| helium | chlorine | neon density | 1.785×10^-4 g/cm^3 | 0.003214 g/cm^3 | 9×10^-4 g/cm^3 molar volume | 22420 cm^3/mol | 11030 cm^3/mol | 22400 cm^3/mol bulk modulus | | 1.1 GPa | refractive index | 1.000035 | 1.000773 | 1.000067 sound speed | 970 m/s | 206 m/s | 936 m/s thermal conductivity | 0.1513 W/(m K) | 0.0089 W/(m K) | 0.0491 W/(m K) (properties at standard conditions)
Electromagnetic properties
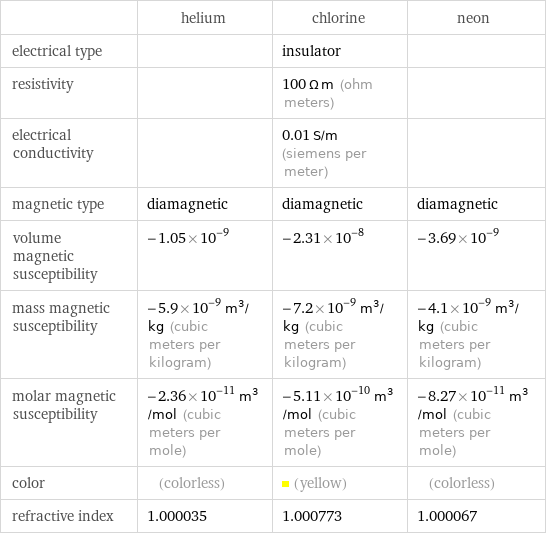
| helium | chlorine | neon electrical type | | insulator | resistivity | | 100 Ω m (ohm meters) | electrical conductivity | | 0.01 S/m (siemens per meter) | magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -1.05×10^-9 | -2.31×10^-8 | -3.69×10^-9 mass magnetic susceptibility | -5.9×10^-9 m^3/kg (cubic meters per kilogram) | -7.2×10^-9 m^3/kg (cubic meters per kilogram) | -4.1×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -2.36×10^-11 m^3/mol (cubic meters per mole) | -5.11×10^-10 m^3/mol (cubic meters per mole) | -8.27×10^-11 m^3/mol (cubic meters per mole) color | (colorless) | (yellow) | (colorless) refractive index | 1.000035 | 1.000773 | 1.000067
Reactivity
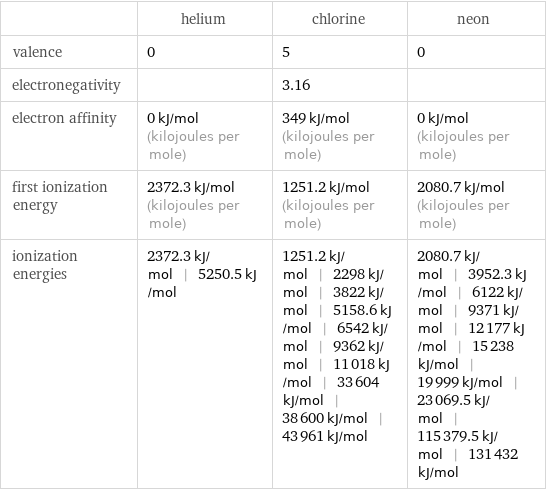
| helium | chlorine | neon valence | 0 | 5 | 0 electronegativity | | 3.16 | electron affinity | 0 kJ/mol (kilojoules per mole) | 349 kJ/mol (kilojoules per mole) | 0 kJ/mol (kilojoules per mole) first ionization energy | 2372.3 kJ/mol (kilojoules per mole) | 1251.2 kJ/mol (kilojoules per mole) | 2080.7 kJ/mol (kilojoules per mole) ionization energies | 2372.3 kJ/mol | 5250.5 kJ/mol | 1251.2 kJ/mol | 2298 kJ/mol | 3822 kJ/mol | 5158.6 kJ/mol | 6542 kJ/mol | 9362 kJ/mol | 11018 kJ/mol | 33604 kJ/mol | 38600 kJ/mol | 43961 kJ/mol | 2080.7 kJ/mol | 3952.3 kJ/mol | 6122 kJ/mol | 9371 kJ/mol | 12177 kJ/mol | 15238 kJ/mol | 19999 kJ/mol | 23069.5 kJ/mol | 115379.5 kJ/mol | 131432 kJ/mol
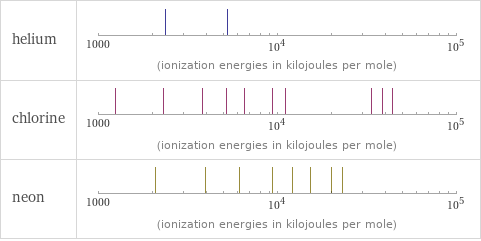
Reactivity
Atomic properties

| helium | chlorine | neon term symbol | ^1S_0 | ^2P_(3/2) | ^1S_0 atomic radius | 31 pm | 100 pm | 38 pm covalent radius | 28 pm | 102 pm | 58 pm van der Waals radius | 140 pm | 175 pm | 154 pm (electronic ground state properties)
Abundances
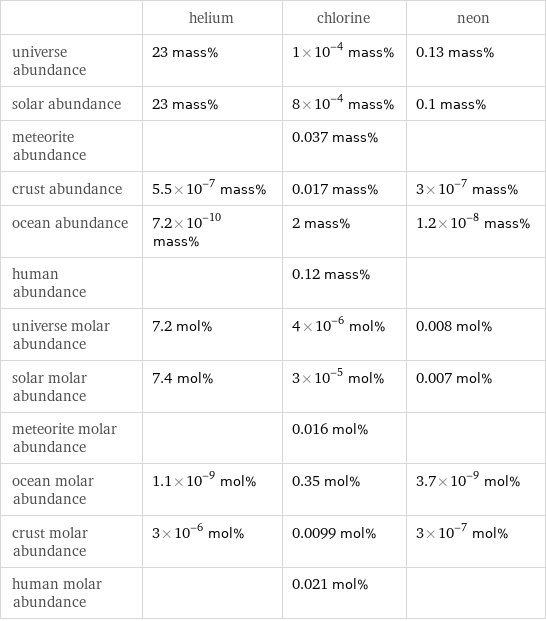
| helium | chlorine | neon universe abundance | 23 mass% | 1×10^-4 mass% | 0.13 mass% solar abundance | 23 mass% | 8×10^-4 mass% | 0.1 mass% meteorite abundance | | 0.037 mass% | crust abundance | 5.5×10^-7 mass% | 0.017 mass% | 3×10^-7 mass% ocean abundance | 7.2×10^-10 mass% | 2 mass% | 1.2×10^-8 mass% human abundance | | 0.12 mass% | universe molar abundance | 7.2 mol% | 4×10^-6 mol% | 0.008 mol% solar molar abundance | 7.4 mol% | 3×10^-5 mol% | 0.007 mol% meteorite molar abundance | | 0.016 mol% | ocean molar abundance | 1.1×10^-9 mol% | 0.35 mol% | 3.7×10^-9 mol% crust molar abundance | 3×10^-6 mol% | 0.0099 mol% | 3×10^-7 mol% human molar abundance | | 0.021 mol% |
Nuclear properties
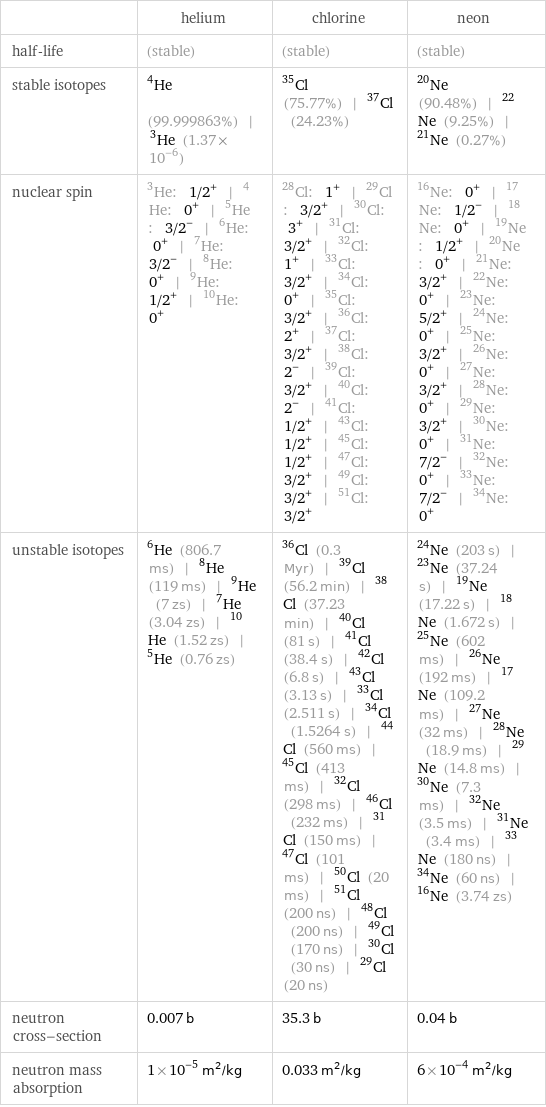
| helium | chlorine | neon half-life | (stable) | (stable) | (stable) stable isotopes | He-4 (99.999863%) | He-3 (1.37×10^-6) | Cl-35 (75.77%) | Cl-37 (24.23%) | Ne-20 (90.48%) | Ne-22 (9.25%) | Ne-21 (0.27%) nuclear spin | He-3: 1/2^+ | He-4: 0^+ | He-5: 3/2^- | He-6: 0^+ | He-7: 3/2^- | He-8: 0^+ | He-9: 1/2^+ | He-10: 0^+ | Cl-28: 1^+ | Cl-29: 3/2^+ | Cl-30: 3^+ | Cl-31: 3/2^+ | Cl-32: 1^+ | Cl-33: 3/2^+ | Cl-34: 0^+ | Cl-35: 3/2^+ | Cl-36: 2^+ | Cl-37: 3/2^+ | Cl-38: 2^- | Cl-39: 3/2^+ | Cl-40: 2^- | Cl-41: 1/2^+ | Cl-43: 1/2^+ | Cl-45: 1/2^+ | Cl-47: 3/2^+ | Cl-49: 3/2^+ | Cl-51: 3/2^+ | Ne-16: 0^+ | Ne-17: 1/2^- | Ne-18: 0^+ | Ne-19: 1/2^+ | Ne-20: 0^+ | Ne-21: 3/2^+ | Ne-22: 0^+ | Ne-23: 5/2^+ | Ne-24: 0^+ | Ne-25: 3/2^+ | Ne-26: 0^+ | Ne-27: 3/2^+ | Ne-28: 0^+ | Ne-29: 3/2^+ | Ne-30: 0^+ | Ne-31: 7/2^- | Ne-32: 0^+ | Ne-33: 7/2^- | Ne-34: 0^+ unstable isotopes | He-6 (806.7 ms) | He-8 (119 ms) | He-9 (7 zs) | He-7 (3.04 zs) | He-10 (1.52 zs) | He-5 (0.76 zs) | Cl-36 (0.3 Myr) | Cl-39 (56.2 min) | Cl-38 (37.23 min) | Cl-40 (81 s) | Cl-41 (38.4 s) | Cl-42 (6.8 s) | Cl-43 (3.13 s) | Cl-33 (2.511 s) | Cl-34 (1.5264 s) | Cl-44 (560 ms) | Cl-45 (413 ms) | Cl-32 (298 ms) | Cl-46 (232 ms) | Cl-31 (150 ms) | Cl-47 (101 ms) | Cl-50 (20 ms) | Cl-51 (200 ns) | Cl-48 (200 ns) | Cl-49 (170 ns) | Cl-30 (30 ns) | Cl-29 (20 ns) | Ne-24 (203 s) | Ne-23 (37.24 s) | Ne-19 (17.22 s) | Ne-18 (1.672 s) | Ne-25 (602 ms) | Ne-26 (192 ms) | Ne-17 (109.2 ms) | Ne-27 (32 ms) | Ne-28 (18.9 ms) | Ne-29 (14.8 ms) | Ne-30 (7.3 ms) | Ne-32 (3.5 ms) | Ne-31 (3.4 ms) | Ne-33 (180 ns) | Ne-34 (60 ns) | Ne-16 (3.74 zs) neutron cross-section | 0.007 b | 35.3 b | 0.04 b neutron mass absorption | 1×10^-5 m^2/kg | 0.033 m^2/kg | 6×10^-4 m^2/kg
Identifiers
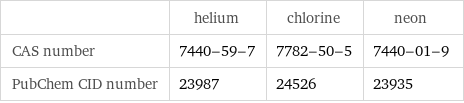
| helium | chlorine | neon CAS number | 7440-59-7 | 7782-50-5 | 7440-01-9 PubChem CID number | 23987 | 24526 | 23935