Input interpretation

halogens | van der Waals radius
Summary
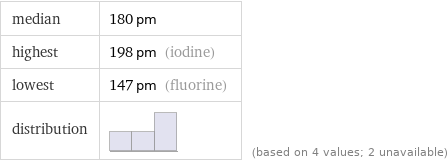
median | 180 pm highest | 198 pm (iodine) lowest | 147 pm (fluorine) distribution | | (based on 4 values; 2 unavailable)
Entities with missing values
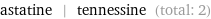
astatine | tennessine (total: 2)
Ranked values
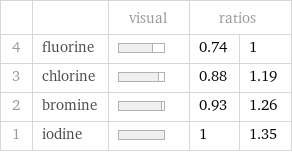
| | visual | ratios | 4 | fluorine | | 0.74 | 1 3 | chlorine | | 0.88 | 1.19 2 | bromine | | 0.93 | 1.26 1 | iodine | | 1 | 1.35
Plot
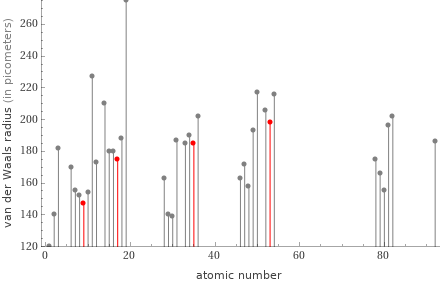
Plot
Periodic table location
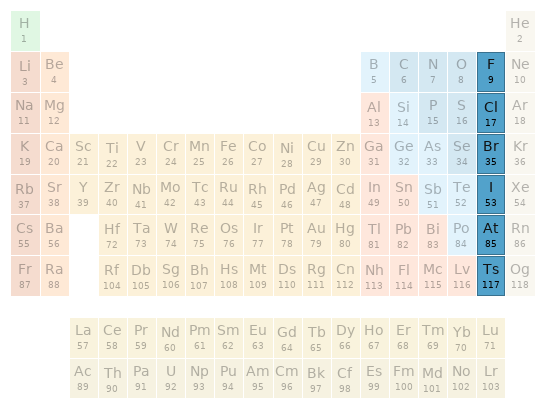
Periodic table location
Atomic radii properties
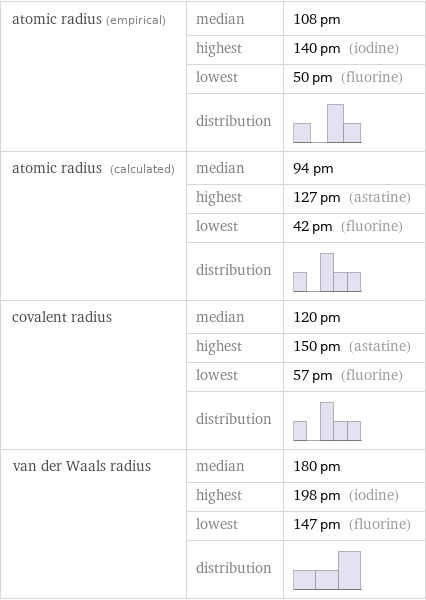
atomic radius (empirical) | median | 108 pm | highest | 140 pm (iodine) | lowest | 50 pm (fluorine) | distribution | atomic radius (calculated) | median | 94 pm | highest | 127 pm (astatine) | lowest | 42 pm (fluorine) | distribution | covalent radius | median | 120 pm | highest | 150 pm (astatine) | lowest | 57 pm (fluorine) | distribution | van der Waals radius | median | 180 pm | highest | 198 pm (iodine) | lowest | 147 pm (fluorine) | distribution |
Units

Van der Waals radius rankings
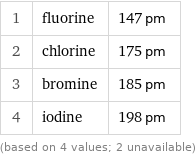
1 | fluorine | 147 pm 2 | chlorine | 175 pm 3 | bromine | 185 pm 4 | iodine | 198 pm (based on 4 values; 2 unavailable)
Unit conversions for median van der Waals radius 180 pm
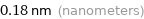
0.18 nm (nanometers)
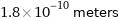
1.8×10^-10 meters
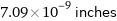
7.09×10^-9 inches
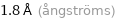
1.8 Å (ångströms)
Comparisons for median van der Waals radius 180 pm
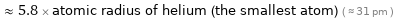
≈ 5.8 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
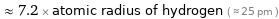
≈ 7.2 × atomic radius of hydrogen ( ≈ 25 pm )