Input interpretation
nickel(II) fluoride | 4-fluoro-1-methyl-2-nitrobenzene
Chemical names and formulas
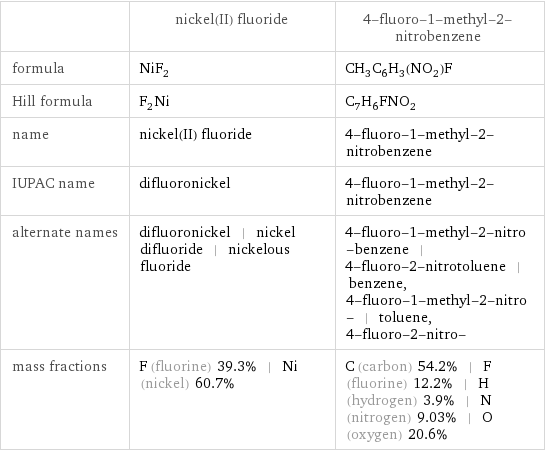
| nickel(II) fluoride | 4-fluoro-1-methyl-2-nitrobenzene formula | NiF_2 | CH_3C_6H_3(NO_2)F Hill formula | F_2Ni | C_7H_6FNO_2 name | nickel(II) fluoride | 4-fluoro-1-methyl-2-nitrobenzene IUPAC name | difluoronickel | 4-fluoro-1-methyl-2-nitrobenzene alternate names | difluoronickel | nickel difluoride | nickelous fluoride | 4-fluoro-1-methyl-2-nitro-benzene | 4-fluoro-2-nitrotoluene | benzene, 4-fluoro-1-methyl-2-nitro- | toluene, 4-fluoro-2-nitro- mass fractions | F (fluorine) 39.3% | Ni (nickel) 60.7% | C (carbon) 54.2% | F (fluorine) 12.2% | H (hydrogen) 3.9% | N (nitrogen) 9.03% | O (oxygen) 20.6%
Structure diagrams
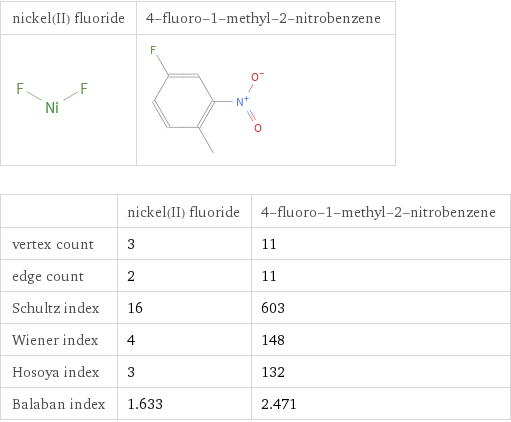
| nickel(II) fluoride | 4-fluoro-1-methyl-2-nitrobenzene vertex count | 3 | 11 edge count | 2 | 11 Schultz index | 16 | 603 Wiener index | 4 | 148 Hosoya index | 3 | 132 Balaban index | 1.633 | 2.471
3D structure
3D structure
Basic properties
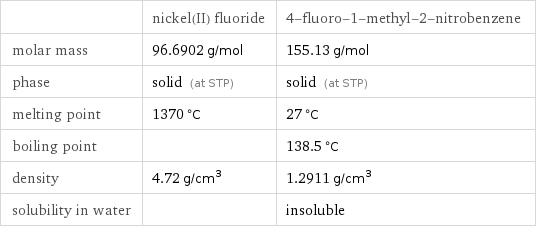
| nickel(II) fluoride | 4-fluoro-1-methyl-2-nitrobenzene molar mass | 96.6902 g/mol | 155.13 g/mol phase | solid (at STP) | solid (at STP) melting point | 1370 °C | 27 °C boiling point | | 138.5 °C density | 4.72 g/cm^3 | 1.2911 g/cm^3 solubility in water | | insoluble
Units

Thermodynamic properties
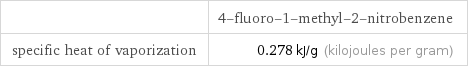
| 4-fluoro-1-methyl-2-nitrobenzene specific heat of vaporization | 0.278 kJ/g (kilojoules per gram)
Solid properties (at STP)
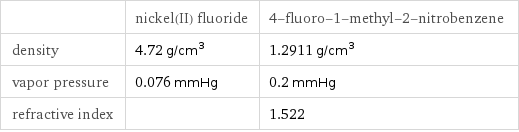
| nickel(II) fluoride | 4-fluoro-1-methyl-2-nitrobenzene density | 4.72 g/cm^3 | 1.2911 g/cm^3 vapor pressure | 0.076 mmHg | 0.2 mmHg refractive index | | 1.522
Units
