Input interpretation
stannic oxide | chlorosyl trifluoride
Chemical names and formulas
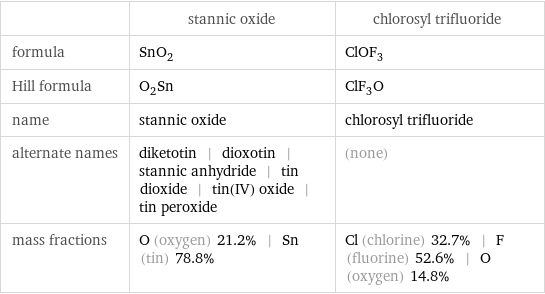
| stannic oxide | chlorosyl trifluoride formula | SnO_2 | ClOF_3 Hill formula | O_2Sn | ClF_3O name | stannic oxide | chlorosyl trifluoride alternate names | diketotin | dioxotin | stannic anhydride | tin dioxide | tin(IV) oxide | tin peroxide | (none) mass fractions | O (oxygen) 21.2% | Sn (tin) 78.8% | Cl (chlorine) 32.7% | F (fluorine) 52.6% | O (oxygen) 14.8%
Structure diagrams

Structure diagrams
Basic properties
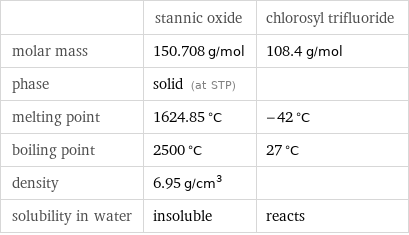
| stannic oxide | chlorosyl trifluoride molar mass | 150.708 g/mol | 108.4 g/mol phase | solid (at STP) | melting point | 1624.85 °C | -42 °C boiling point | 2500 °C | 27 °C density | 6.95 g/cm^3 | solubility in water | insoluble | reacts
Units
