Input interpretation
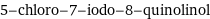
5-chloro-7-iodo-8-quinolinol
Chemical names and formulas
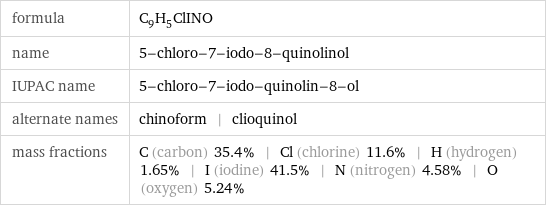
formula | C_9H_5ClINO name | 5-chloro-7-iodo-8-quinolinol IUPAC name | 5-chloro-7-iodo-quinolin-8-ol alternate names | chinoform | clioquinol mass fractions | C (carbon) 35.4% | Cl (chlorine) 11.6% | H (hydrogen) 1.65% | I (iodine) 41.5% | N (nitrogen) 4.58% | O (oxygen) 5.24%
Lewis structure
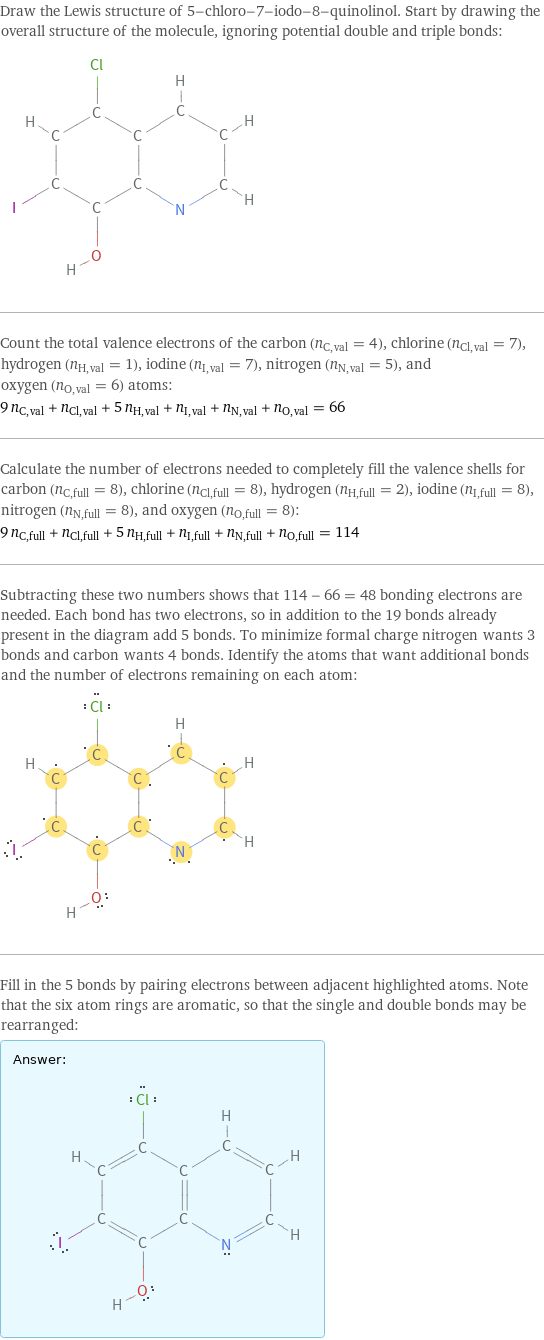
Draw the Lewis structure of 5-chloro-7-iodo-8-quinolinol. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), hydrogen (n_H, val = 1), iodine (n_I, val = 7), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 9 n_C, val + n_Cl, val + 5 n_H, val + n_I, val + n_N, val + n_O, val = 66 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), hydrogen (n_H, full = 2), iodine (n_I, full = 8), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 9 n_C, full + n_Cl, full + 5 n_H, full + n_I, full + n_N, full + n_O, full = 114 Subtracting these two numbers shows that 114 - 66 = 48 bonding electrons are needed. Each bond has two electrons, so in addition to the 19 bonds already present in the diagram add 5 bonds. To minimize formal charge nitrogen wants 3 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 5 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom rings are aromatic, so that the single and double bonds may be rearranged: Answer: | |
3D structure
3D structure
Basic properties
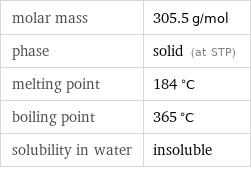
molar mass | 305.5 g/mol phase | solid (at STP) melting point | 184 °C boiling point | 365 °C solubility in water | insoluble
Units

Hydrophobicity and permeability properties

predicted LogP hydrophobicity | 3.66 predicted LogS | -3.06
Basic drug properties

approval status | small molecule | withdrawn drug categories | antifungal agent

brand names | ala-quin | alchloquin | alioform | amebil | amoenol | bactol | barquinol | budoform | chinoform | chinoformum | chlorojodochin | cifoform | cliochinolum | cliquinol | corque | cort-quin | cortin | cremo-quin | dermaform | dioquinol | domeform | domeform-HC | eczecidin | emaform | enteritan | entero-bio form | entero-bioform | entero-septol | entero-vioform | entero-vioformio | enteroquinol | enteroseptol | enterovalodon | enterozol | enterseptol | enterum locorten | entrokin | formtone-HC | Hi-eneterol | Hi-enterol | hydriodide-enterol | hysone | iodenterol | iodoenterol | lekosept | lidaform-HC | loquinol | mycoquin | nioform | nystaform | oralcer | quin-o-creme | quinambicide | quinoform | racet | rheaform | rometin | uad lotion | vioform | vioform-hydrocortisone | vioformio
Chemical identifiers

CAS number | 130-26-7 Beilstein number | 153637 PubChem CID number | 2788 PubChem SID number | 10475089 SMILES identifier | C1=CC2=C(C(=C(C=C2Cl)I)O)N=C1 InChI identifier | InChI=1/C9H5ClINO/c10-6-4-7(11)9(13)8-5(6)2-1-3-12-8/h1-4, 13H InChI key | QCDFBFJGMNKBDO-UHFFFAOYAT EU number | 204-984-4 RTECS number | VC5075000 NSC number | 3531
Toxicity properties

odor | odorless

RTECS classes | drug | mutagen | reproductive effector | human data