Input interpretation
cations | ionic radius
Summary
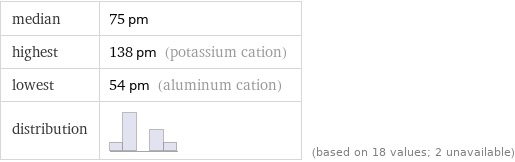
median | 75 pm highest | 138 pm (potassium cation) lowest | 54 pm (aluminum cation) distribution | | (based on 18 values; 2 unavailable)
Entities with missing values
hydrogen cation | dimercury(I) cation (total: 2)
Distribution plots
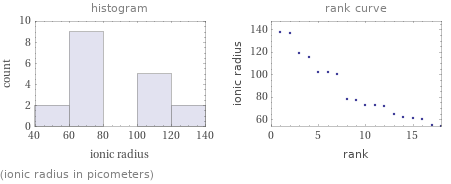
(ionic radius in picometers)
Ionic radius rankings
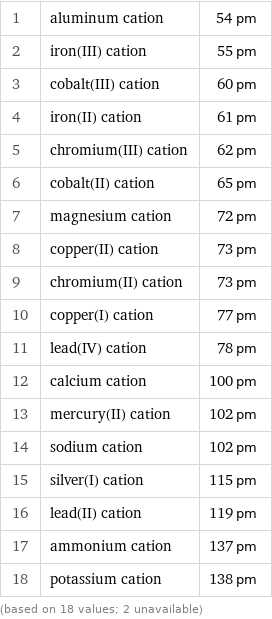
1 | aluminum cation | 54 pm 2 | iron(III) cation | 55 pm 3 | cobalt(III) cation | 60 pm 4 | iron(II) cation | 61 pm 5 | chromium(III) cation | 62 pm 6 | cobalt(II) cation | 65 pm 7 | magnesium cation | 72 pm 8 | copper(II) cation | 73 pm 9 | chromium(II) cation | 73 pm 10 | copper(I) cation | 77 pm 11 | lead(IV) cation | 78 pm 12 | calcium cation | 100 pm 13 | mercury(II) cation | 102 pm 14 | sodium cation | 102 pm 15 | silver(I) cation | 115 pm 16 | lead(II) cation | 119 pm 17 | ammonium cation | 137 pm 18 | potassium cation | 138 pm (based on 18 values; 2 unavailable)
Ionic radii
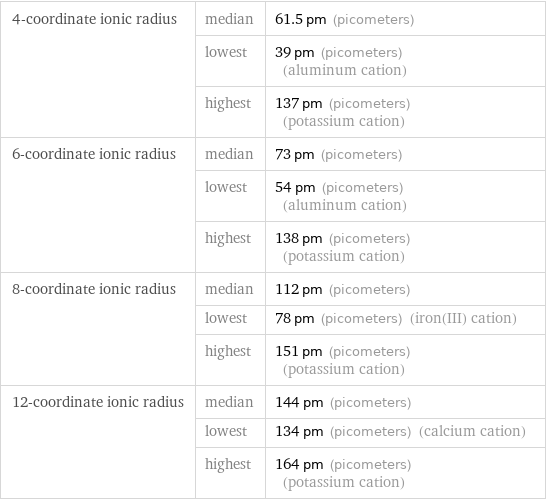
4-coordinate ionic radius | median | 61.5 pm (picometers) | lowest | 39 pm (picometers) (aluminum cation) | highest | 137 pm (picometers) (potassium cation) 6-coordinate ionic radius | median | 73 pm (picometers) | lowest | 54 pm (picometers) (aluminum cation) | highest | 138 pm (picometers) (potassium cation) 8-coordinate ionic radius | median | 112 pm (picometers) | lowest | 78 pm (picometers) (iron(III) cation) | highest | 151 pm (picometers) (potassium cation) 12-coordinate ionic radius | median | 144 pm (picometers) | lowest | 134 pm (picometers) (calcium cation) | highest | 164 pm (picometers) (potassium cation)
Unit conversions for median ionic radius 75 pm

3/40 nm (nanometers)
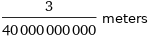
3/40000000000 meters
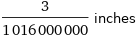
3/1016000000 inches

3/4 Å (ångströms)