Input interpretation

1, 1-bis(3, 4-dimethylphenyl)ethane | is aromatic
Result

True
Aromatic atoms in place
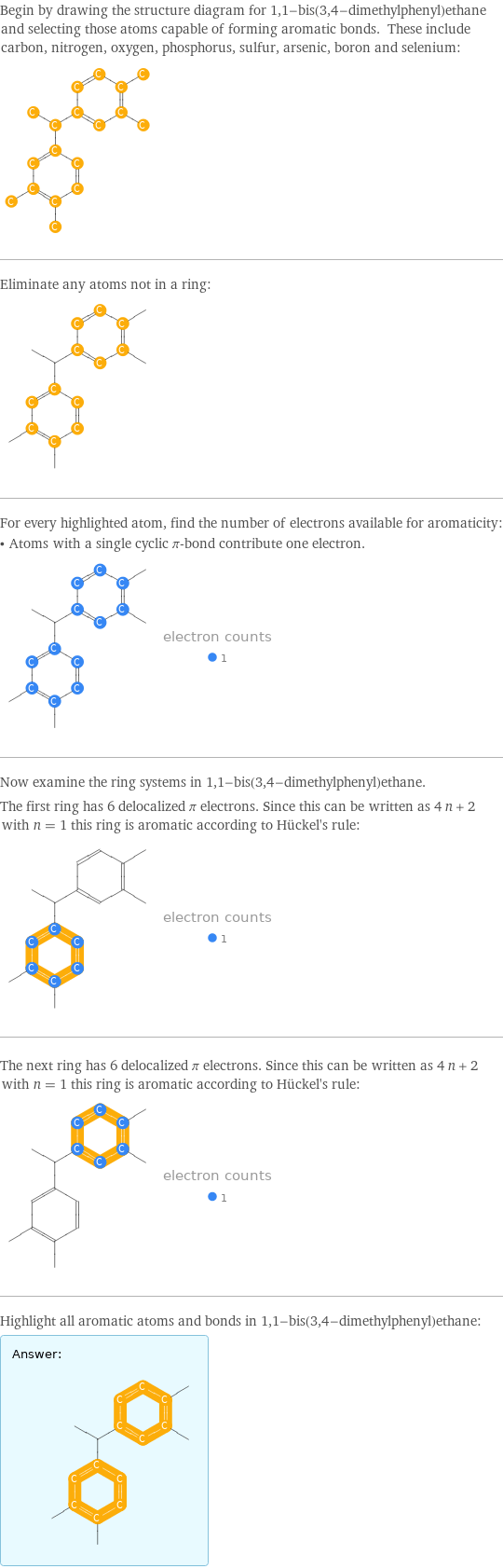
Begin by drawing the structure diagram for 1, 1-bis(3, 4-dimethylphenyl)ethane and selecting those atoms capable of forming aromatic bonds. These include carbon, nitrogen, oxygen, phosphorus, sulfur, arsenic, boron and selenium: Eliminate any atoms not in a ring: For every highlighted atom, find the number of electrons available for aromaticity: • Atoms with a single cyclic π-bond contribute one electron. Now examine the ring systems in 1, 1-bis(3, 4-dimethylphenyl)ethane. The first ring has 6 delocalized π electrons. Since this can be written as 4 n + 2 with n = 1 this ring is aromatic according to Hückel's rule: The next ring has 6 delocalized π electrons. Since this can be written as 4 n + 2 with n = 1 this ring is aromatic according to Hückel's rule: Highlight all aromatic atoms and bonds in 1, 1-bis(3, 4-dimethylphenyl)ethane: Answer: | |