Input interpretation

solid compounds | element types
Summary

aluminum | beryllium | carbon | calcium | cobalt | copper | fluorine | iron | hydrogen | potassium | lithium | magnesium | nitrogen | sodium | oxygen | scandium | silicon | vanadium | zinc
Periodic table location
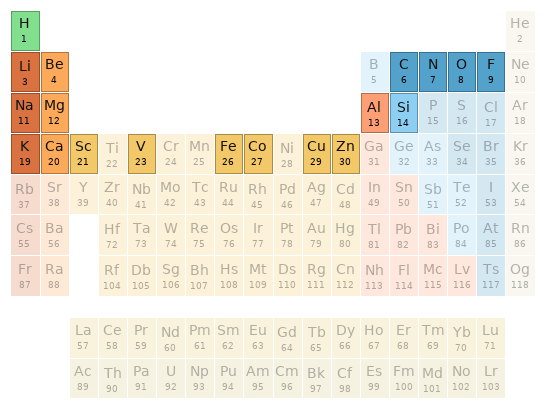
Periodic table location
Images

Images
Basic elemental properties
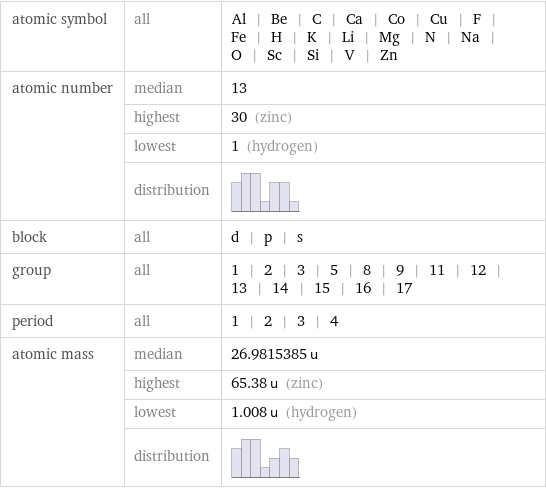
atomic symbol | all | Al | Be | C | Ca | Co | Cu | F | Fe | H | K | Li | Mg | N | Na | O | Sc | Si | V | Zn atomic number | median | 13 | highest | 30 (zinc) | lowest | 1 (hydrogen) | distribution | block | all | d | p | s group | all | 1 | 2 | 3 | 5 | 8 | 9 | 11 | 12 | 13 | 14 | 15 | 16 | 17 period | all | 1 | 2 | 3 | 4 atomic mass | median | 26.9815385 u | highest | 65.38 u (zinc) | lowest | 1.008 u (hydrogen) | distribution |
Table
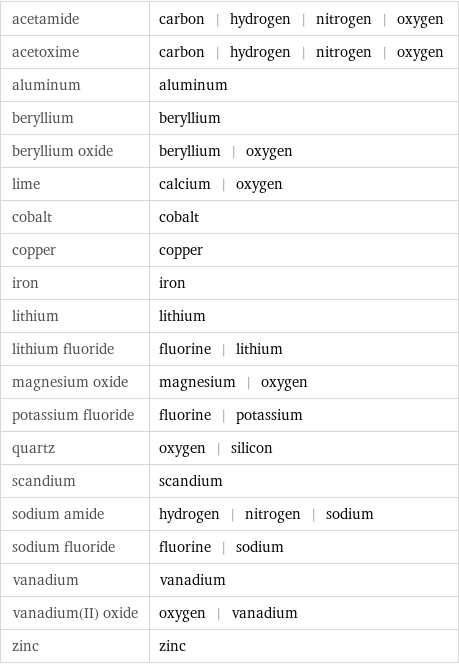
acetamide | carbon | hydrogen | nitrogen | oxygen acetoxime | carbon | hydrogen | nitrogen | oxygen aluminum | aluminum beryllium | beryllium beryllium oxide | beryllium | oxygen lime | calcium | oxygen cobalt | cobalt copper | copper iron | iron lithium | lithium lithium fluoride | fluorine | lithium magnesium oxide | magnesium | oxygen potassium fluoride | fluorine | potassium quartz | oxygen | silicon scandium | scandium sodium amide | hydrogen | nitrogen | sodium sodium fluoride | fluorine | sodium vanadium | vanadium vanadium(II) oxide | oxygen | vanadium zinc | zinc
Thermodynamic properties
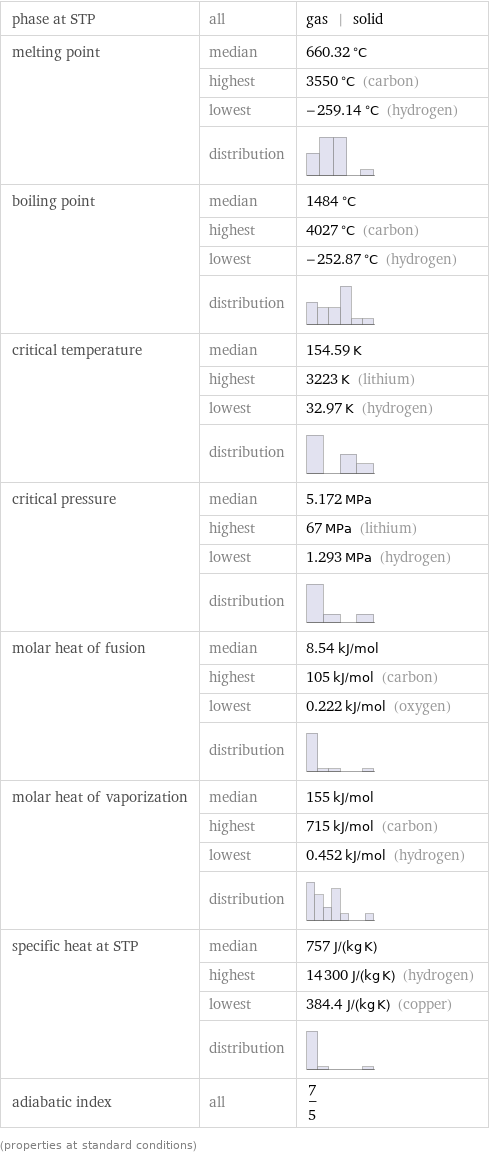
phase at STP | all | gas | solid melting point | median | 660.32 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 1484 °C | highest | 4027 °C (carbon) | lowest | -252.87 °C (hydrogen) | distribution | critical temperature | median | 154.59 K | highest | 3223 K (lithium) | lowest | 32.97 K (hydrogen) | distribution | critical pressure | median | 5.172 MPa | highest | 67 MPa (lithium) | lowest | 1.293 MPa (hydrogen) | distribution | molar heat of fusion | median | 8.54 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.222 kJ/mol (oxygen) | distribution | molar heat of vaporization | median | 155 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 0.452 kJ/mol (hydrogen) | distribution | specific heat at STP | median | 757 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 384.4 J/(kg K) (copper) | distribution | adiabatic index | all | 7/5 (properties at standard conditions)
Material properties
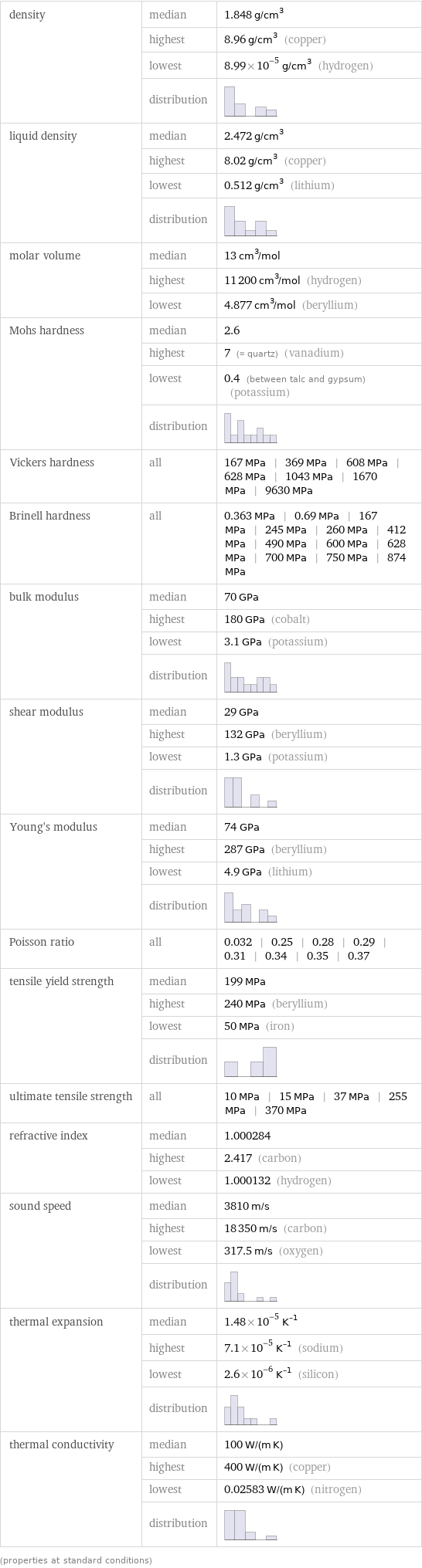
density | median | 1.848 g/cm^3 | highest | 8.96 g/cm^3 (copper) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 2.472 g/cm^3 | highest | 8.02 g/cm^3 (copper) | lowest | 0.512 g/cm^3 (lithium) | distribution | molar volume | median | 13 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 4.877 cm^3/mol (beryllium) Mohs hardness | median | 2.6 | highest | 7 (≈ quartz) (vanadium) | lowest | 0.4 (between talc and gypsum) (potassium) | distribution | Vickers hardness | all | 167 MPa | 369 MPa | 608 MPa | 628 MPa | 1043 MPa | 1670 MPa | 9630 MPa Brinell hardness | all | 0.363 MPa | 0.69 MPa | 167 MPa | 245 MPa | 260 MPa | 412 MPa | 490 MPa | 600 MPa | 628 MPa | 700 MPa | 750 MPa | 874 MPa bulk modulus | median | 70 GPa | highest | 180 GPa (cobalt) | lowest | 3.1 GPa (potassium) | distribution | shear modulus | median | 29 GPa | highest | 132 GPa (beryllium) | lowest | 1.3 GPa (potassium) | distribution | Young's modulus | median | 74 GPa | highest | 287 GPa (beryllium) | lowest | 4.9 GPa (lithium) | distribution | Poisson ratio | all | 0.032 | 0.25 | 0.28 | 0.29 | 0.31 | 0.34 | 0.35 | 0.37 tensile yield strength | median | 199 MPa | highest | 240 MPa (beryllium) | lowest | 50 MPa (iron) | distribution | ultimate tensile strength | all | 10 MPa | 15 MPa | 37 MPa | 255 MPa | 370 MPa refractive index | median | 1.000284 | highest | 2.417 (carbon) | lowest | 1.000132 (hydrogen) sound speed | median | 3810 m/s | highest | 18350 m/s (carbon) | lowest | 317.5 m/s (oxygen) | distribution | thermal expansion | median | 1.48×10^-5 K^(-1) | highest | 7.1×10^-5 K^(-1) (sodium) | lowest | 2.6×10^-6 K^(-1) (silicon) | distribution | thermal conductivity | median | 100 W/(m K) | highest | 400 W/(m K) (copper) | lowest | 0.02583 W/(m K) (nitrogen) | distribution | (properties at standard conditions)
Electromagnetic properties
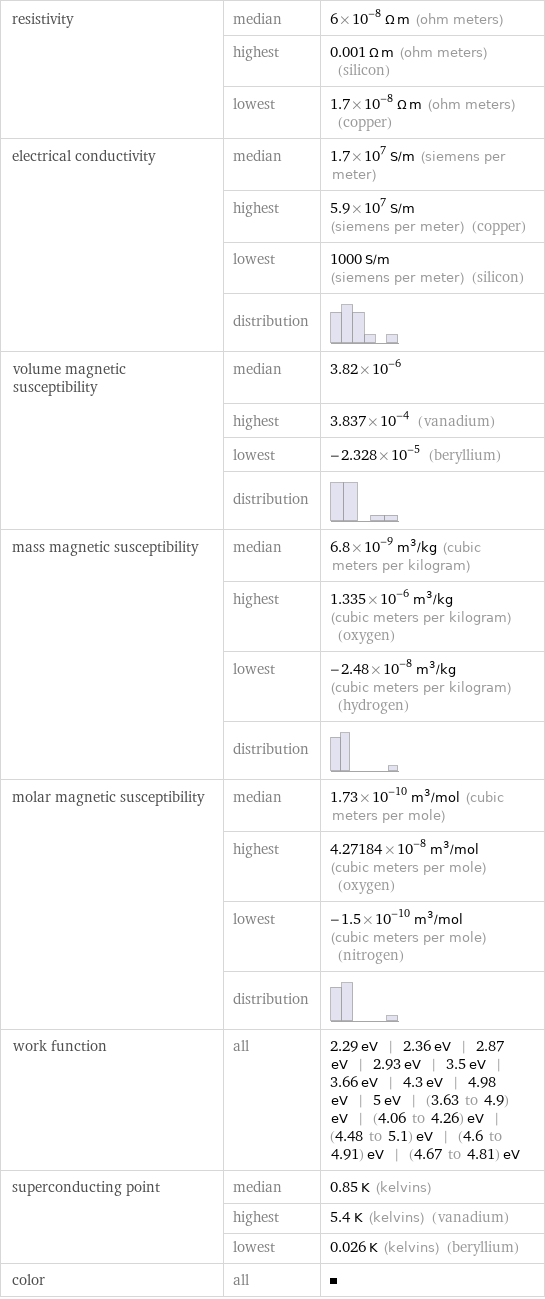
resistivity | median | 6×10^-8 Ω m (ohm meters) | highest | 0.001 Ω m (ohm meters) (silicon) | lowest | 1.7×10^-8 Ω m (ohm meters) (copper) electrical conductivity | median | 1.7×10^7 S/m (siemens per meter) | highest | 5.9×10^7 S/m (siemens per meter) (copper) | lowest | 1000 S/m (siemens per meter) (silicon) | distribution | volume magnetic susceptibility | median | 3.82×10^-6 | highest | 3.837×10^-4 (vanadium) | lowest | -2.328×10^-5 (beryllium) | distribution | mass magnetic susceptibility | median | 6.8×10^-9 m^3/kg (cubic meters per kilogram) | highest | 1.335×10^-6 m^3/kg (cubic meters per kilogram) (oxygen) | lowest | -2.48×10^-8 m^3/kg (cubic meters per kilogram) (hydrogen) | distribution | molar magnetic susceptibility | median | 1.73×10^-10 m^3/mol (cubic meters per mole) | highest | 4.27184×10^-8 m^3/mol (cubic meters per mole) (oxygen) | lowest | -1.5×10^-10 m^3/mol (cubic meters per mole) (nitrogen) | distribution | work function | all | 2.29 eV | 2.36 eV | 2.87 eV | 2.93 eV | 3.5 eV | 3.66 eV | 4.3 eV | 4.98 eV | 5 eV | (3.63 to 4.9) eV | (4.06 to 4.26) eV | (4.48 to 5.1) eV | (4.6 to 4.91) eV | (4.67 to 4.81) eV superconducting point | median | 0.85 K (kelvins) | highest | 5.4 K (kelvins) (vanadium) | lowest | 0.026 K (kelvins) (beryllium) color | all |
Reactivity
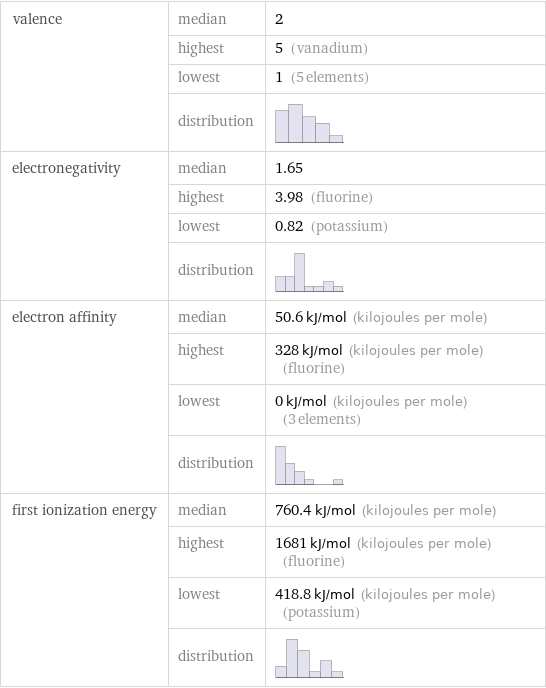
valence | median | 2 | highest | 5 (vanadium) | lowest | 1 (5 elements) | distribution | electronegativity | median | 1.65 | highest | 3.98 (fluorine) | lowest | 0.82 (potassium) | distribution | electron affinity | median | 50.6 kJ/mol (kilojoules per mole) | highest | 328 kJ/mol (kilojoules per mole) (fluorine) | lowest | 0 kJ/mol (kilojoules per mole) (3 elements) | distribution | first ionization energy | median | 760.4 kJ/mol (kilojoules per mole) | highest | 1681 kJ/mol (kilojoules per mole) (fluorine) | lowest | 418.8 kJ/mol (kilojoules per mole) (potassium) | distribution |