Input interpretation
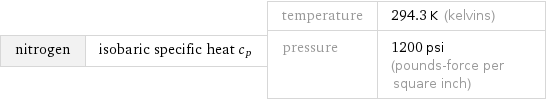
nitrogen | isobaric specific heat c_p | temperature | 294.3 K (kelvins) pressure | 1200 psi (pounds-force per square inch)
Result
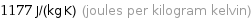
1177 J/(kg K) (joules per kilogram kelvin)
Interpretation
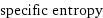
specific entropy
Basic unit dimensions
![[length]^2 [time]^(-2) [temperature]^(-1)](../image_source/1e1b1fc51f88175b94f7ba2f11f553ed.png)
[length]^2 [time]^(-2) [temperature]^(-1)
Thermodynamic properties
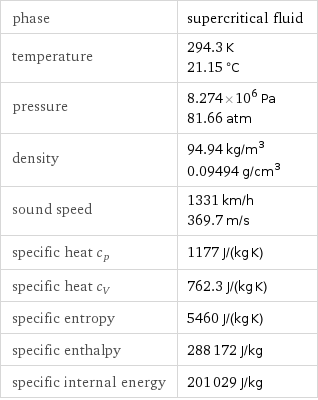
phase | supercritical fluid temperature | 294.3 K 21.15 °C pressure | 8.274×10^6 Pa 81.66 atm density | 94.94 kg/m^3 0.09494 g/cm^3 sound speed | 1331 km/h 369.7 m/s specific heat c_p | 1177 J/(kg K) specific heat c_V | 762.3 J/(kg K) specific entropy | 5460 J/(kg K) specific enthalpy | 288172 J/kg specific internal energy | 201029 J/kg
Phase diagram
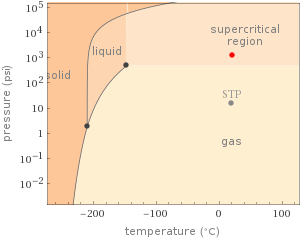
Phase diagram