Input interpretation
deuterated iodomethane (D3)
Chemical names and formulas
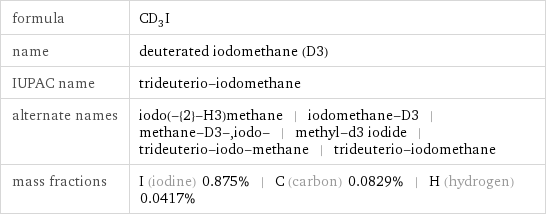
formula | CD_3I name | deuterated iodomethane (D3) IUPAC name | trideuterio-iodomethane alternate names | iodo(-{2}-H3)methane | iodomethane-D3 | methane-D3-, iodo- | methyl-d3 iodide | trideuterio-iodo-methane | trideuterio-iodomethane mass fractions | I (iodine) 0.875% | C (carbon) 0.0829% | H (hydrogen) 0.0417%
Lewis structure
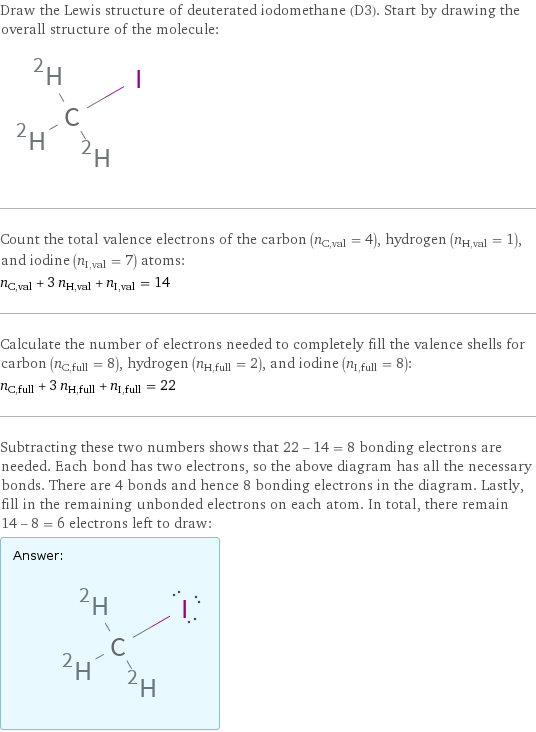
Draw the Lewis structure of deuterated iodomethane (D3). Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and iodine (n_I, val = 7) atoms: n_C, val + 3 n_H, val + n_I, val = 14 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and iodine (n_I, full = 8): n_C, full + 3 n_H, full + n_I, full = 22 Subtracting these two numbers shows that 22 - 14 = 8 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 4 bonds and hence 8 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 14 - 8 = 6 electrons left to draw: Answer: | |
3D structure

3D structure
Basic properties
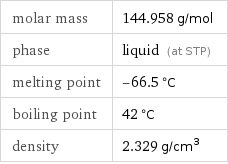
molar mass | 144.958 g/mol phase | liquid (at STP) melting point | -66.5 °C boiling point | 42 °C density | 2.329 g/cm^3
Units

Liquid properties (at STP)
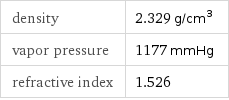
density | 2.329 g/cm^3 vapor pressure | 1177 mmHg refractive index | 1.526
Units

Non-standard atom properties

H-2 | 3
Chemical identifiers
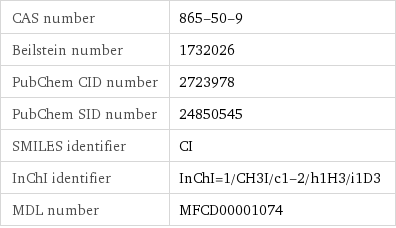
CAS number | 865-50-9 Beilstein number | 1732026 PubChem CID number | 2723978 PubChem SID number | 24850545 SMILES identifier | CI InChI identifier | InChI=1/CH3I/c1-2/h1H3/i1D3 MDL number | MFCD00001074
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0