Input interpretation
alkali metals | specific heat of vaporization
Summary
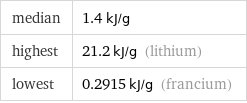
median | 1.4 kJ/g highest | 21.2 kJ/g (lithium) lowest | 0.2915 kJ/g (francium)
Units

Ranked values
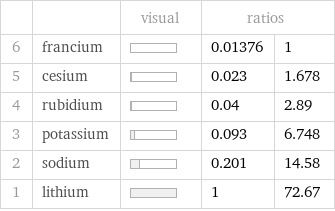
| | visual | ratios | 6 | francium | | 0.01376 | 1 5 | cesium | | 0.023 | 1.678 4 | rubidium | | 0.04 | 2.89 3 | potassium | | 0.093 | 6.748 2 | sodium | | 0.201 | 14.58 1 | lithium | | 1 | 72.67
Specific heat of vaporization rankings
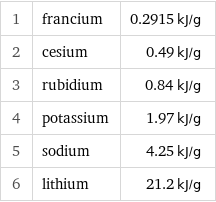
1 | francium | 0.2915 kJ/g 2 | cesium | 0.49 kJ/g 3 | rubidium | 0.84 kJ/g 4 | potassium | 1.97 kJ/g 5 | sodium | 4.25 kJ/g 6 | lithium | 21.2 kJ/g
Unit conversions for median specific heat of vaporization 1.4 kJ/g
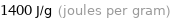
1400 J/g (joules per gram)
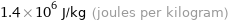
1.4×10^6 J/kg (joules per kilogram)

340 cal_th/g (thermochemical calories per gram)
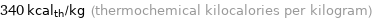
340 kcal_th/kg (thermochemical kilocalories per kilogram)
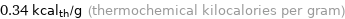
0.34 kcal_th/g (thermochemical kilocalories per gram)

600 BTU_IT/lb (IT British thermal units per pound)