Input interpretation

interstellar molecules | vapor pressure
Summary
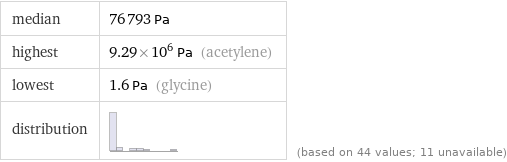
median | 76793 Pa highest | 9.29×10^6 Pa (acetylene) lowest | 1.6 Pa (glycine) distribution | | (based on 44 values; 11 unavailable)
Entities with missing values
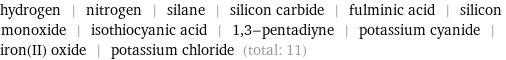
hydrogen | nitrogen | silane | silicon carbide | fulminic acid | silicon monoxide | isothiocyanic acid | 1, 3-pentadiyne | potassium cyanide | iron(II) oxide | potassium chloride (total: 11)
Distribution plots
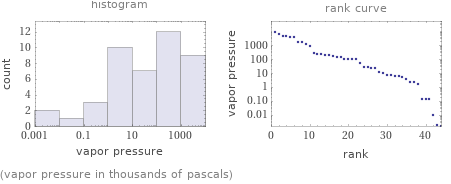
(vapor pressure in thousands of pascals)
Vapor pressure rankings
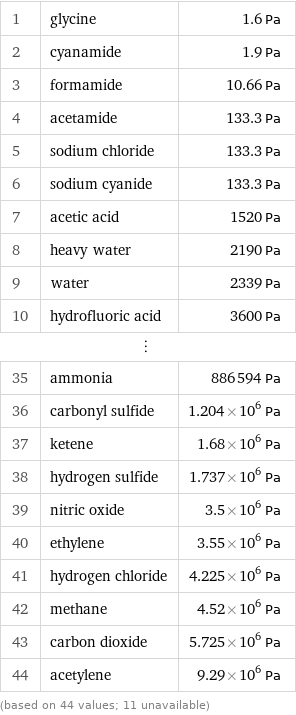
1 | glycine | 1.6 Pa 2 | cyanamide | 1.9 Pa 3 | formamide | 10.66 Pa 4 | acetamide | 133.3 Pa 5 | sodium chloride | 133.3 Pa 6 | sodium cyanide | 133.3 Pa 7 | acetic acid | 1520 Pa 8 | heavy water | 2190 Pa 9 | water | 2339 Pa 10 | hydrofluoric acid | 3600 Pa ⋮ | | 35 | ammonia | 886594 Pa 36 | carbonyl sulfide | 1.204×10^6 Pa 37 | ketene | 1.68×10^6 Pa 38 | hydrogen sulfide | 1.737×10^6 Pa 39 | nitric oxide | 3.5×10^6 Pa 40 | ethylene | 3.55×10^6 Pa 41 | hydrogen chloride | 4.225×10^6 Pa 42 | methane | 4.52×10^6 Pa 43 | carbon dioxide | 5.725×10^6 Pa 44 | acetylene | 9.29×10^6 Pa (based on 44 values; 11 unavailable)
Unit conversions for median vapor pressure 76793 Pa
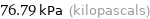
76.79 kPa (kilopascals)
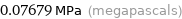
0.07679 MPa (megapascals)

76793 N/m^2 (newtons per square meter)
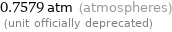
0.7579 atm (atmospheres) (unit officially deprecated)

0.7831 at (technical atmospheres) (unit officially deprecated)
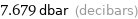
7.679 dbar (decibars)
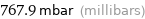
767.9 mbar (millibars)
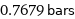
0.7679 bars
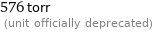
576 torr (unit officially deprecated)
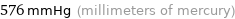
576 mmHg (millimeters of mercury)
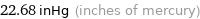
22.68 inHg (inches of mercury)
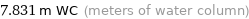
7.831 m WC (meters of water column)

783.1 cm WC (centimeters of water column)

308.3 in WC (inches of water column (conventional))
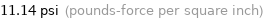
11.14 psi (pounds-force per square inch)
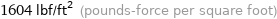
1604 lbf/ft^2 (pounds-force per square foot)
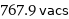
767.9 vacs
Comparisons for median vapor pressure 76793 Pa
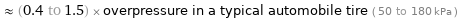
≈ (0.4 to 1.5) × overpressure in a typical automobile tire ( 50 to 180 kPa )
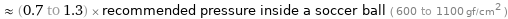
≈ (0.7 to 1.3) × recommended pressure inside a soccer ball ( 600 to 1100 gf/cm^2 )

≈ 1.1 × pressure inside an incandescent light bulb ( ≈ 70000 Pa )
Corresponding quantities
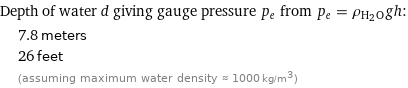
Depth of water d giving gauge pressure p_e from p_e = ρ_(H_2O)gh: | 7.8 meters | 26 feet | (assuming maximum water density ≈ 1000 kg/m^3)

Standard Atmosphere altitude: | 2.3 km (kilometers) | 1.4 miles | 7475 feet | (based on 1976 US Standard Atmosphere)