Input interpretation
nickel carbonate | sodium hexafluoroarsenate(V)
Chemical names and formulas
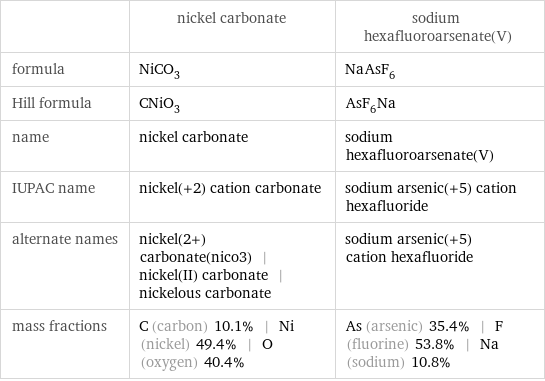
| nickel carbonate | sodium hexafluoroarsenate(V) formula | NiCO_3 | NaAsF_6 Hill formula | CNiO_3 | AsF_6Na name | nickel carbonate | sodium hexafluoroarsenate(V) IUPAC name | nickel(+2) cation carbonate | sodium arsenic(+5) cation hexafluoride alternate names | nickel(2+) carbonate(nico3) | nickel(II) carbonate | nickelous carbonate | sodium arsenic(+5) cation hexafluoride mass fractions | C (carbon) 10.1% | Ni (nickel) 49.4% | O (oxygen) 40.4% | As (arsenic) 35.4% | F (fluorine) 53.8% | Na (sodium) 10.8%
Structure diagrams
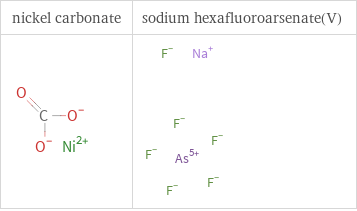
Structure diagrams
Basic properties
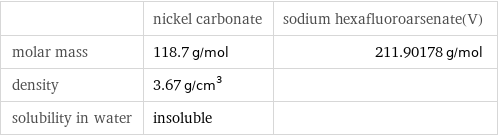
| nickel carbonate | sodium hexafluoroarsenate(V) molar mass | 118.7 g/mol | 211.90178 g/mol density | 3.67 g/cm^3 | solubility in water | insoluble |
Units
