Input information

Maxwell speed distribution probability | temperature | 1200 K (kelvins) mass of molecule | flerovium (chemical element) (atomic mass): 289 u (unified atomic mass units) minimum velocity | 500 m/s (meters per second) maximum velocity | 1000 m/s (meters per second)
Equation
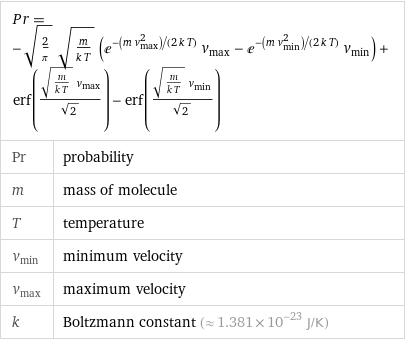
Pr = -sqrt(2/π) sqrt(m/(k T)) (e^(-(m v_max^2)/(2 k T)) v_max - e^(-(m v_min^2)/(2 k T)) v_min) + erf((sqrt(m/(k T)) v_max)/sqrt(2)) - erf((sqrt(m/(k T)) v_min)/sqrt(2)) | | Pr | probability m | mass of molecule T | temperature v_min | minimum velocity v_max | maximum velocity k | Boltzmann constant (≈ 1.381×10^-23 J/K)
Result

probability | 0.065
Velocity equations
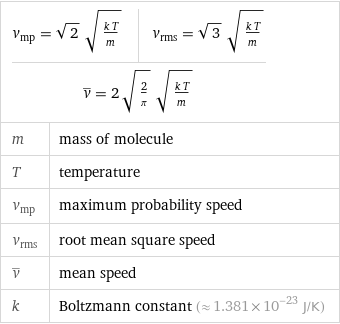
v_mp = sqrt(2) sqrt((k T)/m) | v_rms = sqrt(3) sqrt((k T)/m) v^_ = 2 sqrt(2/π) sqrt((k T)/m) | | m | mass of molecule T | temperature v_mp | maximum probability speed v_rms | root mean square speed v^_ | mean speed k | Boltzmann constant (≈ 1.381×10^-23 J/K)
Units

Velocity parameters
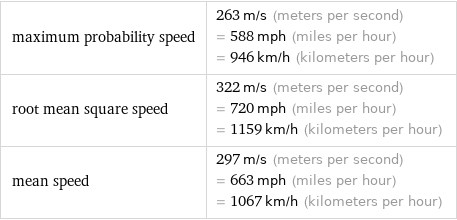
maximum probability speed | 263 m/s (meters per second) = 588 mph (miles per hour) = 946 km/h (kilometers per hour) root mean square speed | 322 m/s (meters per second) = 720 mph (miles per hour) = 1159 km/h (kilometers per hour) mean speed | 297 m/s (meters per second) = 663 mph (miles per hour) = 1067 km/h (kilometers per hour)
Probability density function
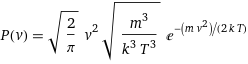
P(v) = sqrt(2/π) v^2 sqrt(m^3/(k^3 T^3)) e^(-(m v^2)/(2 k T))
Probability density vs. speed
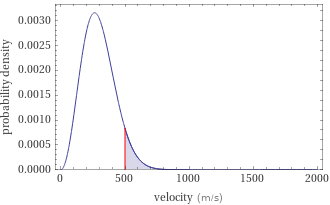
Probability density vs. speed
Units
