Input interpretation

2 ethylhexanoate | formal charges
Result
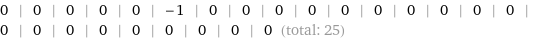
0 | 0 | 0 | 0 | 0 | -1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (total: 25)
Lewis structure
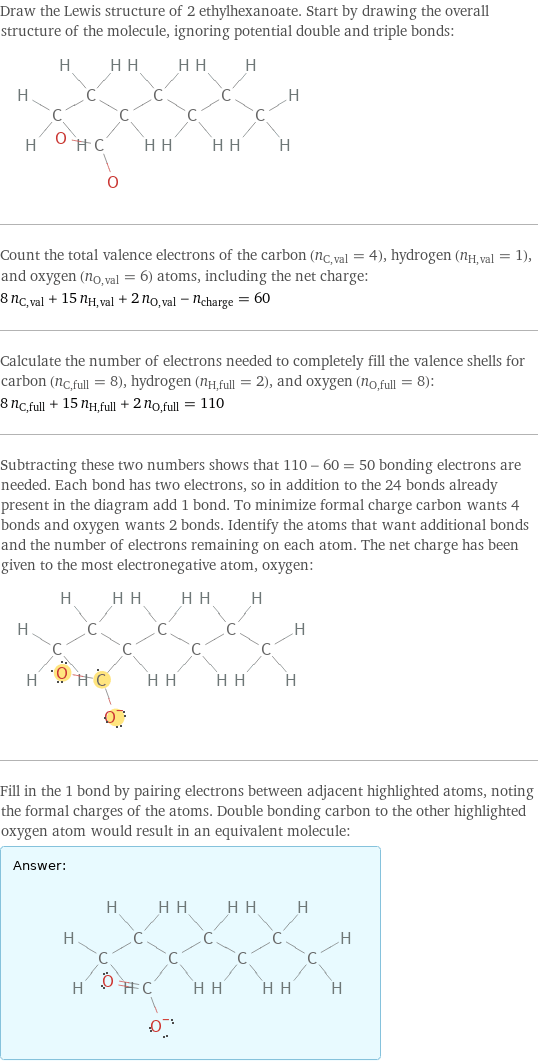
Draw the Lewis structure of 2 ethylhexanoate. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms, including the net charge: 8 n_C, val + 15 n_H, val + 2 n_O, val - n_charge = 60 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): 8 n_C, full + 15 n_H, full + 2 n_O, full = 110 Subtracting these two numbers shows that 110 - 60 = 50 bonding electrons are needed. Each bond has two electrons, so in addition to the 24 bonds already present in the diagram add 1 bond. To minimize formal charge carbon wants 4 bonds and oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom. The net charge has been given to the most electronegative atom, oxygen: Fill in the 1 bond by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms. Double bonding carbon to the other highlighted oxygen atom would result in an equivalent molecule: Answer: | |