Input interpretation
alkaline earth metals | molar heat of vaporization
Summary
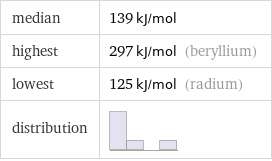
median | 139 kJ/mol highest | 297 kJ/mol (beryllium) lowest | 125 kJ/mol (radium) distribution |
Units

Ranked values
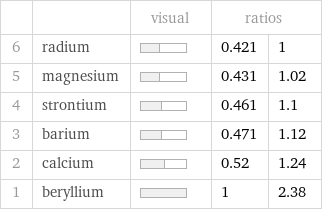
| | visual | ratios | 6 | radium | | 0.421 | 1 5 | magnesium | | 0.431 | 1.02 4 | strontium | | 0.461 | 1.1 3 | barium | | 0.471 | 1.12 2 | calcium | | 0.52 | 1.24 1 | beryllium | | 1 | 2.38
Molar heat of vaporization rankings
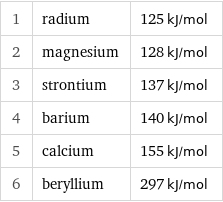
1 | radium | 125 kJ/mol 2 | magnesium | 128 kJ/mol 3 | strontium | 137 kJ/mol 4 | barium | 140 kJ/mol 5 | calcium | 155 kJ/mol 6 | beryllium | 297 kJ/mol
Unit conversions for median molar heat of vaporization 139 kJ/mol
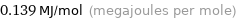
0.139 MJ/mol (megajoules per mole)
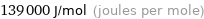
139000 J/mol (joules per mole)

33.1 kcal/mol (thermochemical kilocalories per mole)
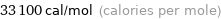
33100 cal/mol (calories per mole)
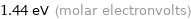
1.44 eV (molar electronvolts)