Input interpretation
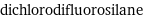
dichlorodifluorosilane
Chemical names and formulas

formula | SiCl_2F_2 Hill formula | Cl_2F_2Si_1 name | dichlorodifluorosilane mass fractions | Cl (chlorine) 51.8% | F (fluorine) 27.7% | Si (silicon) 20.5%
Lewis structure
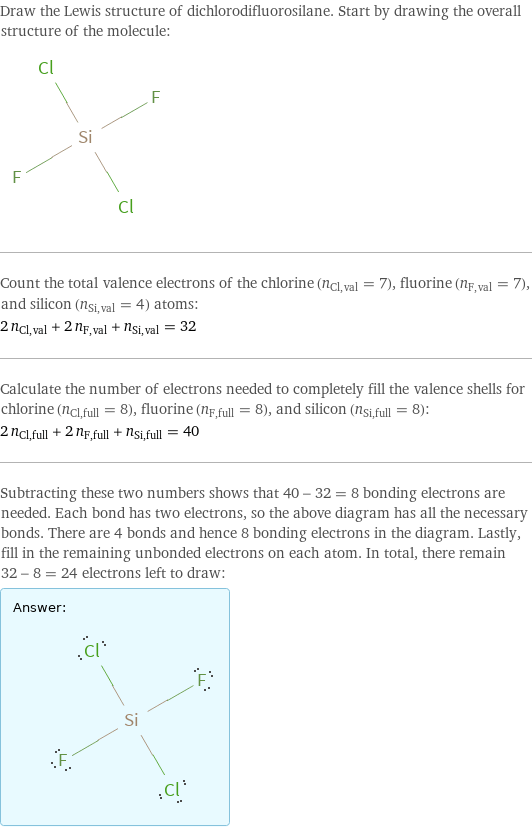
Draw the Lewis structure of dichlorodifluorosilane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the chlorine (n_Cl, val = 7), fluorine (n_F, val = 7), and silicon (n_Si, val = 4) atoms: 2 n_Cl, val + 2 n_F, val + n_Si, val = 32 Calculate the number of electrons needed to completely fill the valence shells for chlorine (n_Cl, full = 8), fluorine (n_F, full = 8), and silicon (n_Si, full = 8): 2 n_Cl, full + 2 n_F, full + n_Si, full = 40 Subtracting these two numbers shows that 40 - 32 = 8 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 4 bonds and hence 8 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 32 - 8 = 24 electrons left to draw: Answer: | |
Basic properties
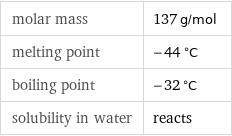
molar mass | 137 g/mol melting point | -44 °C boiling point | -32 °C solubility in water | reacts
Units

Chemical identifiers
(F)F](../image_source/690801f1e45131069050b5bf020f1fa2.png)
CAS number | 18356-71-3 SMILES identifier | Cl[Si](Cl)(F)F