Input interpretation
selenium hexafluoride | nickel(II) fluoride
Chemical names and formulas
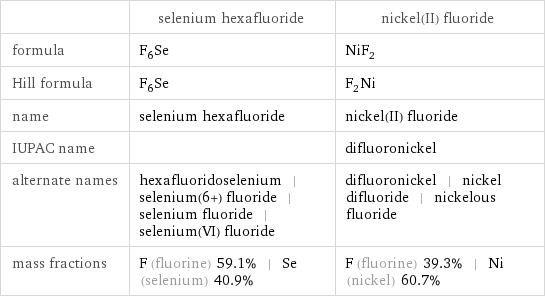
| selenium hexafluoride | nickel(II) fluoride formula | F_6Se | NiF_2 Hill formula | F_6Se | F_2Ni name | selenium hexafluoride | nickel(II) fluoride IUPAC name | | difluoronickel alternate names | hexafluoridoselenium | selenium(6+) fluoride | selenium fluoride | selenium(VI) fluoride | difluoronickel | nickel difluoride | nickelous fluoride mass fractions | F (fluorine) 59.1% | Se (selenium) 40.9% | F (fluorine) 39.3% | Ni (nickel) 60.7%
Structure diagrams
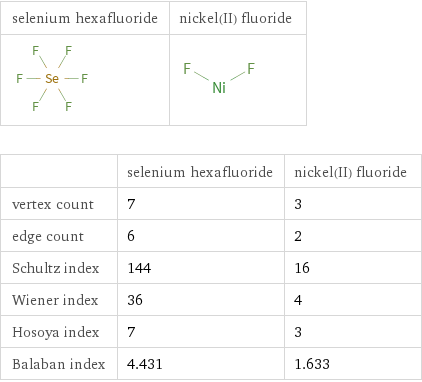
| selenium hexafluoride | nickel(II) fluoride vertex count | 7 | 3 edge count | 6 | 2 Schultz index | 144 | 16 Wiener index | 36 | 4 Hosoya index | 7 | 3 Balaban index | 4.431 | 1.633
Basic properties
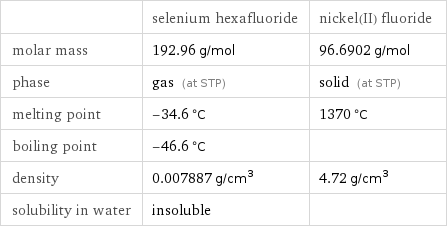
| selenium hexafluoride | nickel(II) fluoride molar mass | 192.96 g/mol | 96.6902 g/mol phase | gas (at STP) | solid (at STP) melting point | -34.6 °C | 1370 °C boiling point | -46.6 °C | density | 0.007887 g/cm^3 | 4.72 g/cm^3 solubility in water | insoluble |
Units

Gas properties
| selenium hexafluoride density | 0.007887 g/cm^3 vapor density | 6.7 molar volume | 24470 cm^3/mol
Units
