Input interpretation
nitrogen dioxide | butane
Chemical names and formulas
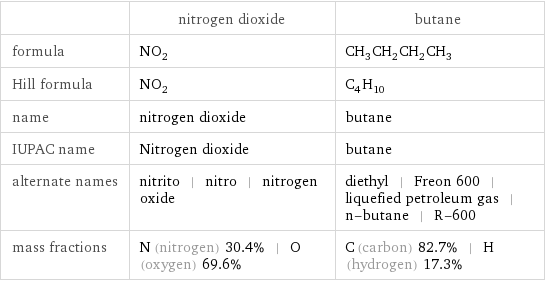
| nitrogen dioxide | butane formula | NO_2 | CH_3CH_2CH_2CH_3 Hill formula | NO_2 | C_4H_10 name | nitrogen dioxide | butane IUPAC name | Nitrogen dioxide | butane alternate names | nitrito | nitro | nitrogen oxide | diethyl | Freon 600 | liquefied petroleum gas | n-butane | R-600 mass fractions | N (nitrogen) 30.4% | O (oxygen) 69.6% | C (carbon) 82.7% | H (hydrogen) 17.3%
Structure diagrams
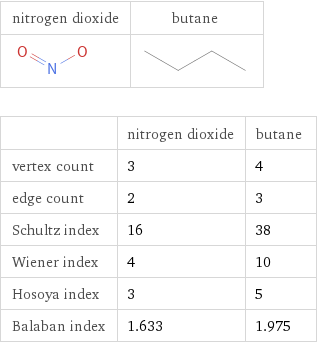
| nitrogen dioxide | butane vertex count | 3 | 4 edge count | 2 | 3 Schultz index | 16 | 38 Wiener index | 4 | 10 Hosoya index | 3 | 5 Balaban index | 1.633 | 1.975
3D structure
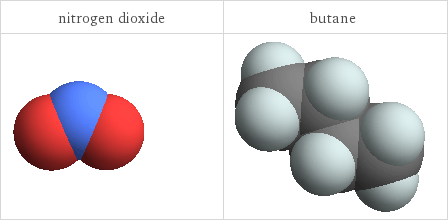
3D structure
Basic properties
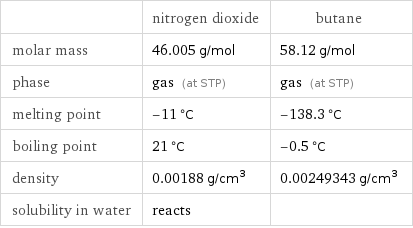
| nitrogen dioxide | butane molar mass | 46.005 g/mol | 58.12 g/mol phase | gas (at STP) | gas (at STP) melting point | -11 °C | -138.3 °C boiling point | 21 °C | -0.5 °C density | 0.00188 g/cm^3 | 0.00249343 g/cm^3 solubility in water | reacts |
Units

Hydrophobicity and permeability properties

| butane predicted LogP hydrophobicity | 2.81 predicted LogS | -1.91
Gas properties (at STP)
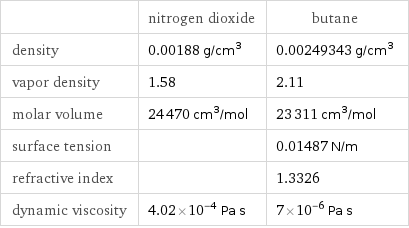
| nitrogen dioxide | butane density | 0.00188 g/cm^3 | 0.00249343 g/cm^3 vapor density | 1.58 | 2.11 molar volume | 24470 cm^3/mol | 23311 cm^3/mol surface tension | | 0.01487 N/m refractive index | | 1.3326 dynamic viscosity | 4.02×10^-4 Pa s | 7×10^-6 Pa s
Units
