Input interpretation
strontium oxide | ironate
Chemical names and formulas
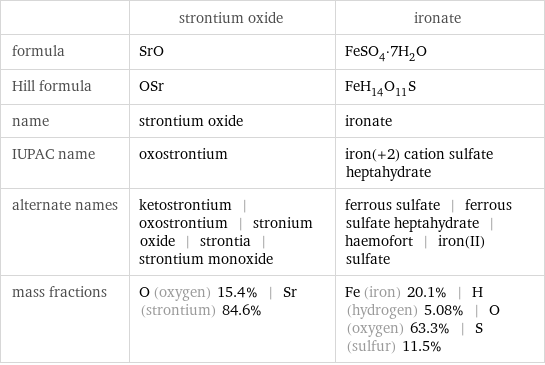
| strontium oxide | ironate formula | SrO | FeSO_4·7H_2O Hill formula | OSr | FeH_14O_11S name | strontium oxide | ironate IUPAC name | oxostrontium | iron(+2) cation sulfate heptahydrate alternate names | ketostrontium | oxostrontium | stronium oxide | strontia | strontium monoxide | ferrous sulfate | ferrous sulfate heptahydrate | haemofort | iron(II) sulfate mass fractions | O (oxygen) 15.4% | Sr (strontium) 84.6% | Fe (iron) 20.1% | H (hydrogen) 5.08% | O (oxygen) 63.3% | S (sulfur) 11.5%
Structure diagrams
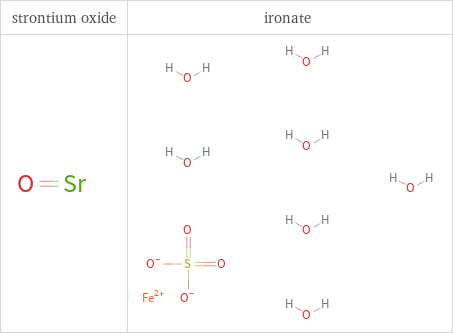
Structure diagrams
Basic properties
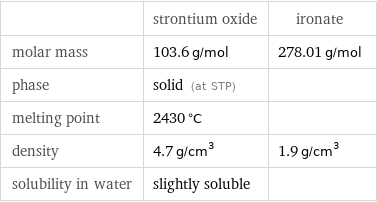
| strontium oxide | ironate molar mass | 103.6 g/mol | 278.01 g/mol phase | solid (at STP) | melting point | 2430 °C | density | 4.7 g/cm^3 | 1.9 g/cm^3 solubility in water | slightly soluble |
Units
