Input interpretation
stannous chloride dihydrate | cobalt(II) selenite dihydrate
Chemical names and formulas
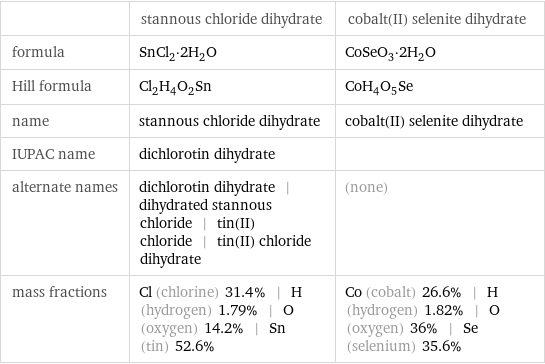
| stannous chloride dihydrate | cobalt(II) selenite dihydrate formula | SnCl_2·2H_2O | CoSeO_3·2H_2O Hill formula | Cl_2H_4O_2Sn | CoH_4O_5Se name | stannous chloride dihydrate | cobalt(II) selenite dihydrate IUPAC name | dichlorotin dihydrate | alternate names | dichlorotin dihydrate | dihydrated stannous chloride | tin(II) chloride | tin(II) chloride dihydrate | (none) mass fractions | Cl (chlorine) 31.4% | H (hydrogen) 1.79% | O (oxygen) 14.2% | Sn (tin) 52.6% | Co (cobalt) 26.6% | H (hydrogen) 1.82% | O (oxygen) 36% | Se (selenium) 35.6%
Structure diagrams
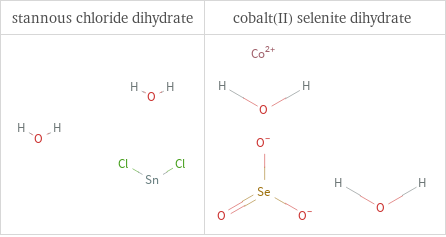
Structure diagrams
Basic properties
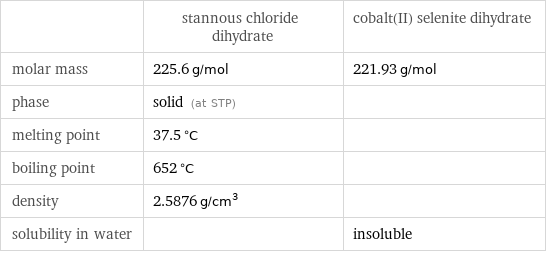
| stannous chloride dihydrate | cobalt(II) selenite dihydrate molar mass | 225.6 g/mol | 221.93 g/mol phase | solid (at STP) | melting point | 37.5 °C | boiling point | 652 °C | density | 2.5876 g/cm^3 | solubility in water | | insoluble
Units
