Input interpretation
phosphorus pentachloride | scandium
Chemical names and formulas
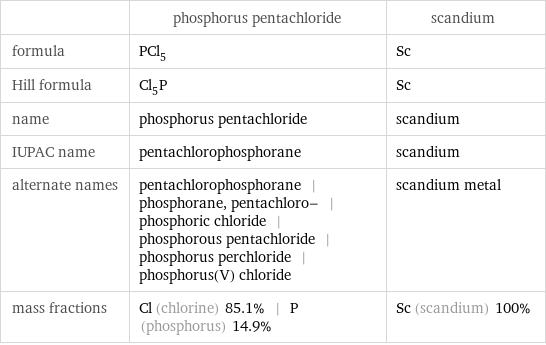
| phosphorus pentachloride | scandium formula | PCl_5 | Sc Hill formula | Cl_5P | Sc name | phosphorus pentachloride | scandium IUPAC name | pentachlorophosphorane | scandium alternate names | pentachlorophosphorane | phosphorane, pentachloro- | phosphoric chloride | phosphorous pentachloride | phosphorus perchloride | phosphorus(V) chloride | scandium metal mass fractions | Cl (chlorine) 85.1% | P (phosphorus) 14.9% | Sc (scandium) 100%
Structure diagrams

Structure diagrams
Basic properties
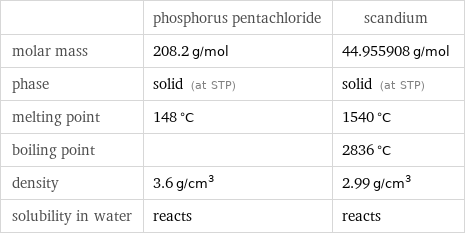
| phosphorus pentachloride | scandium molar mass | 208.2 g/mol | 44.955908 g/mol phase | solid (at STP) | solid (at STP) melting point | 148 °C | 1540 °C boiling point | | 2836 °C density | 3.6 g/cm^3 | 2.99 g/cm^3 solubility in water | reacts | reacts
Units

Thermodynamic properties
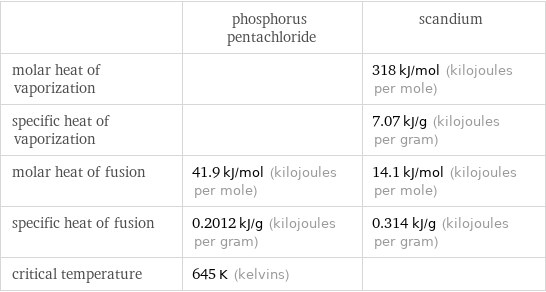
| phosphorus pentachloride | scandium molar heat of vaporization | | 318 kJ/mol (kilojoules per mole) specific heat of vaporization | | 7.07 kJ/g (kilojoules per gram) molar heat of fusion | 41.9 kJ/mol (kilojoules per mole) | 14.1 kJ/mol (kilojoules per mole) specific heat of fusion | 0.2012 kJ/g (kilojoules per gram) | 0.314 kJ/g (kilojoules per gram) critical temperature | 645 K (kelvins) |
Solid properties (at STP)
| phosphorus pentachloride | scandium density | 3.6 g/cm^3 | 2.99 g/cm^3
Units

Chemical identifiers
![| phosphorus pentachloride | scandium CAS number | 10026-13-8 | 7440-20-2 PubChem CID number | 24819 | 23952 PubChem SID number | | 24855799 SMILES identifier | P(Cl)(Cl)(Cl)(Cl)Cl | [Sc]](../image_source/b62fe84b4744df97c1c3cdc0b238840a.png)
| phosphorus pentachloride | scandium CAS number | 10026-13-8 | 7440-20-2 PubChem CID number | 24819 | 23952 PubChem SID number | | 24855799 SMILES identifier | P(Cl)(Cl)(Cl)(Cl)Cl | [Sc]
NFPA label
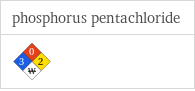
NFPA label