Input interpretation
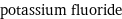
potassium fluoride
Chemical names and formulas

formula | KF Hill formula | FK name | potassium fluoride alternate names | fluorure de potassium | potassium fluoride (1:1) | potassium fluoride anhydrous mass fractions | F (fluorine) 32.7% | K (potassium) 67.3%
Structure diagram
Structure diagram
Basic properties
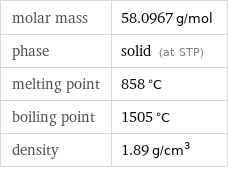
molar mass | 58.0967 g/mol phase | solid (at STP) melting point | 858 °C boiling point | 1505 °C density | 1.89 g/cm^3
Units

Solid properties (at STP)
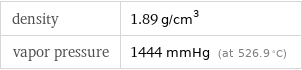
density | 1.89 g/cm^3 vapor pressure | 1444 mmHg (at 526.9 °C)
Units

Thermodynamic properties
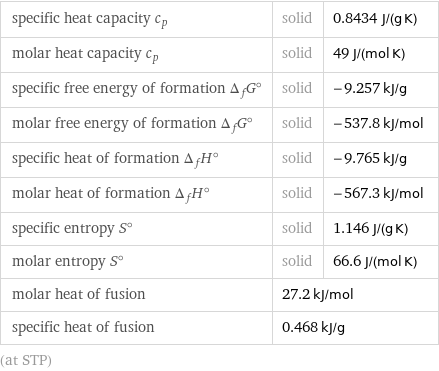
specific heat capacity c_p | solid | 0.8434 J/(g K) molar heat capacity c_p | solid | 49 J/(mol K) specific free energy of formation Δ_fG° | solid | -9.257 kJ/g molar free energy of formation Δ_fG° | solid | -537.8 kJ/mol specific heat of formation Δ_fH° | solid | -9.765 kJ/g molar heat of formation Δ_fH° | solid | -567.3 kJ/mol specific entropy S° | solid | 1.146 J/(g K) molar entropy S° | solid | 66.6 J/(mol K) molar heat of fusion | 27.2 kJ/mol | specific heat of fusion | 0.468 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7789-23-3 Beilstein number | 3902818 PubChem CID number | 522689 PubChem SID number | 24853749 SMILES identifier | [F-].[K+] InChI identifier | InChI=1/FH.K/h1H;/q;+1/p-1/fF.K/h1h;/q-1;m RTECS number | TT0700000 MDL number | MFCD00011398](../image_source/213baf502e182501d078de6186fd8dea.png)
CAS number | 7789-23-3 Beilstein number | 3902818 PubChem CID number | 522689 PubChem SID number | 24853749 SMILES identifier | [F-].[K+] InChI identifier | InChI=1/FH.K/h1H;/q;+1/p-1/fF.K/h1h;/q-1;m RTECS number | TT0700000 MDL number | MFCD00011398
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 1 NFPA reactivity rating | 0
Toxicity properties
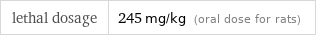
lethal dosage | 245 mg/kg (oral dose for rats)

probable lethal dose for man | 30 mL (milliliters) RTECS classes | mutagen | reproductive effector
Ion equivalents

K^+ (potassium cation) | 1 F^- (fluoride anion) | 1