Input interpretation
rubidium nitrate
Chemical names and formulas

formula | RbNO_3 Hill formula | NO_3Rb name | rubidium nitrate IUPAC name | rubidium(+1) cation nitrate alternate names | nitric acid, rubidium salt | rubidium(+1) cation nitrate | rubidiumnitratewhitextl mass fractions | N (nitrogen) 9.5% | O (oxygen) 32.5% | Rb (rubidium) 58%
Structure diagram
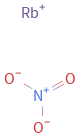
Structure diagram
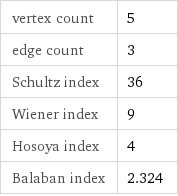
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
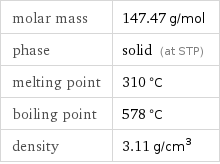
molar mass | 147.47 g/mol phase | solid (at STP) melting point | 310 °C boiling point | 578 °C density | 3.11 g/cm^3
Units

Solid properties (at STP)

density | 3.11 g/cm^3 refractive index | 1.524
Units

Thermodynamic properties
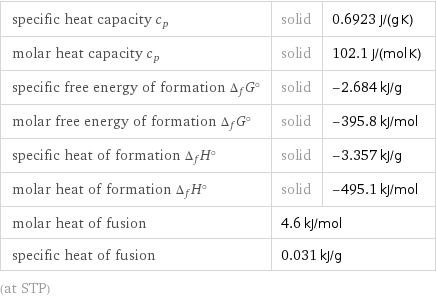
specific heat capacity c_p | solid | 0.6923 J/(g K) molar heat capacity c_p | solid | 102.1 J/(mol K) specific free energy of formation Δ_fG° | solid | -2.684 kJ/g molar free energy of formation Δ_fG° | solid | -395.8 kJ/mol specific heat of formation Δ_fH° | solid | -3.357 kJ/g molar heat of formation Δ_fH° | solid | -495.1 kJ/mol molar heat of fusion | 4.6 kJ/mol | specific heat of fusion | 0.031 kJ/g | (at STP)
Chemical identifiers
([O-])[O-].[Rb+] InChI identifier | InChI=1/NO3.Rb/c2-1(3)4;/q-1;+1 RTECS number | QV0900000 MDL number | MFCD00011193](../image_source/f6877c4378450044b43e9ca0fddf6e75.png)
CAS number | 13126-12-0 PubChem CID number | 25731 PubChem SID number | 24852247 SMILES identifier | [N+](=O)([O-])[O-].[Rb+] InChI identifier | InChI=1/NO3.Rb/c2-1(3)4;/q-1;+1 RTECS number | QV0900000 MDL number | MFCD00011193
NFPA label

NFPA label

NFPA hazards | oxidizing agent
Toxicity properties

RTECS classes | other
Ion equivalents

Rb^+ (rubidium cation) | 1 (NO_3)^- (nitrate anion) | 1