Input interpretation
polar aprotic solvents
Members
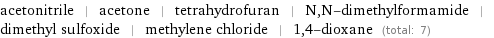
acetonitrile | acetone | tetrahydrofuran | N, N-dimethylformamide | dimethyl sulfoxide | methylene chloride | 1, 4-dioxane (total: 7)
Structure diagrams
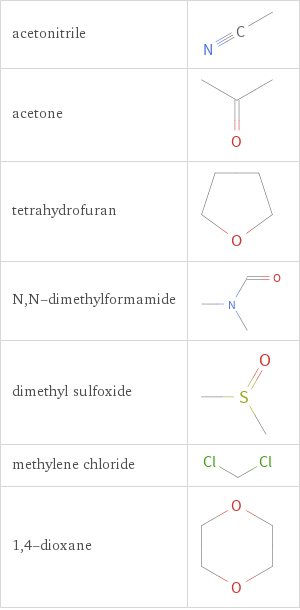
Structure diagrams
3D structure
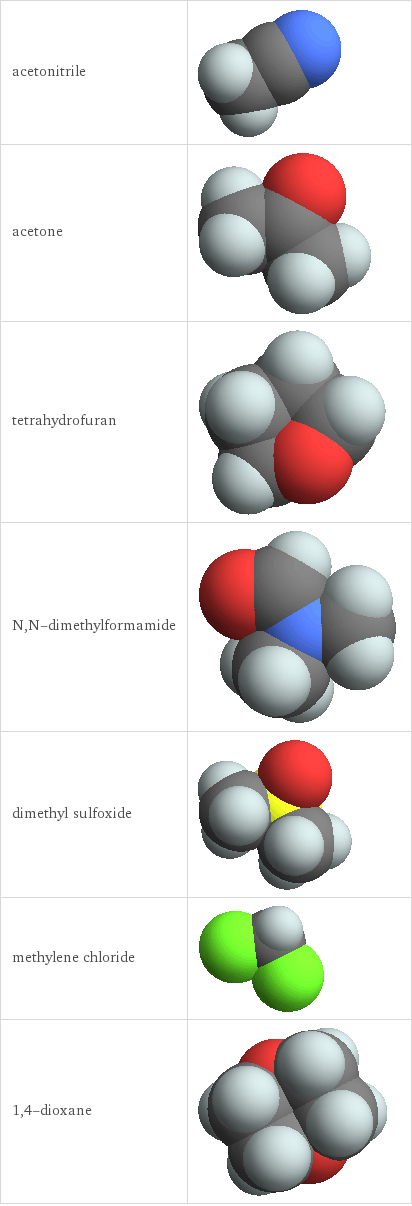
3D structure
Basic properties
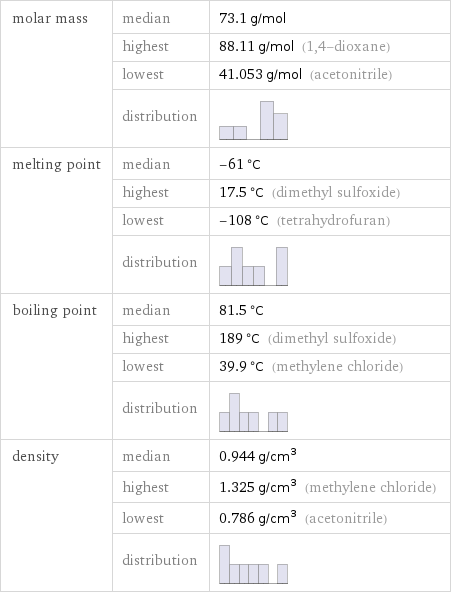
molar mass | median | 73.1 g/mol | highest | 88.11 g/mol (1, 4-dioxane) | lowest | 41.053 g/mol (acetonitrile) | distribution | melting point | median | -61 °C | highest | 17.5 °C (dimethyl sulfoxide) | lowest | -108 °C (tetrahydrofuran) | distribution | boiling point | median | 81.5 °C | highest | 189 °C (dimethyl sulfoxide) | lowest | 39.9 °C (methylene chloride) | distribution | density | median | 0.944 g/cm^3 | highest | 1.325 g/cm^3 (methylene chloride) | lowest | 0.786 g/cm^3 (acetonitrile) | distribution |