Input interpretation
vanadium(II) oxide
Chemical names and formulas

formula | OV name | vanadium(II) oxide IUPAC name | oxovanadium alternate names | vanadium monoxide | vanadium oxide | vanadium oxide (1:1) mass fractions | O (oxygen) 23.9% | V (vanadium) 76.1%
Structure diagram
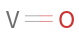
Structure diagram
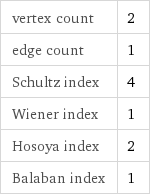
vertex count | 2 edge count | 1 Schultz index | 4 Wiener index | 1 Hosoya index | 2 Balaban index | 1
Basic properties
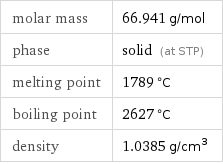
molar mass | 66.941 g/mol phase | solid (at STP) melting point | 1789 °C boiling point | 2627 °C density | 1.0385 g/cm^3
Units

Solid properties (at STP)
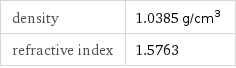
density | 1.0385 g/cm^3 refractive index | 1.5763
Units

Thermodynamic properties
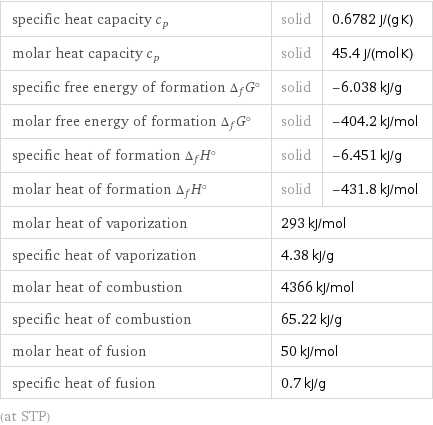
specific heat capacity c_p | solid | 0.6782 J/(g K) molar heat capacity c_p | solid | 45.4 J/(mol K) specific free energy of formation Δ_fG° | solid | -6.038 kJ/g molar free energy of formation Δ_fG° | solid | -404.2 kJ/mol specific heat of formation Δ_fH° | solid | -6.451 kJ/g molar heat of formation Δ_fH° | solid | -431.8 kJ/mol molar heat of vaporization | 293 kJ/mol | specific heat of vaporization | 4.38 kJ/g | molar heat of combustion | 4366 kJ/mol | specific heat of combustion | 65.22 kJ/g | molar heat of fusion | 50 kJ/mol | specific heat of fusion | 0.7 kJ/g | (at STP)
Chemical identifiers
![CAS number | 12035-98-2 PubChem CID number | 24411 SMILES identifier | O=[V]](../image_source/d72c64800d0286502f7d278356756c58.png)
CAS number | 12035-98-2 PubChem CID number | 24411 SMILES identifier | O=[V]