Input interpretation
potassium ferrate(VI) | cobalt(II) bromate hexahydrate
Chemical names and formulas
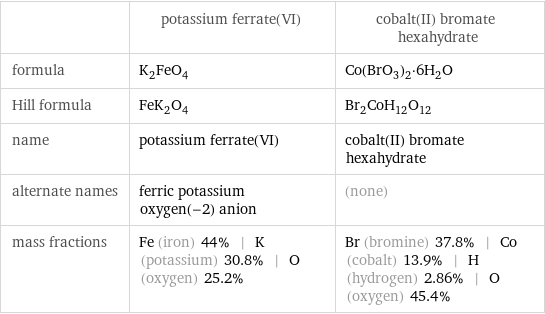
| potassium ferrate(VI) | cobalt(II) bromate hexahydrate formula | K_2FeO_4 | Co(BrO_3)_2·6H_2O Hill formula | FeK_2O_4 | Br_2CoH_12O_12 name | potassium ferrate(VI) | cobalt(II) bromate hexahydrate alternate names | ferric potassium oxygen(-2) anion | (none) mass fractions | Fe (iron) 44% | K (potassium) 30.8% | O (oxygen) 25.2% | Br (bromine) 37.8% | Co (cobalt) 13.9% | H (hydrogen) 2.86% | O (oxygen) 45.4%
Structure diagrams
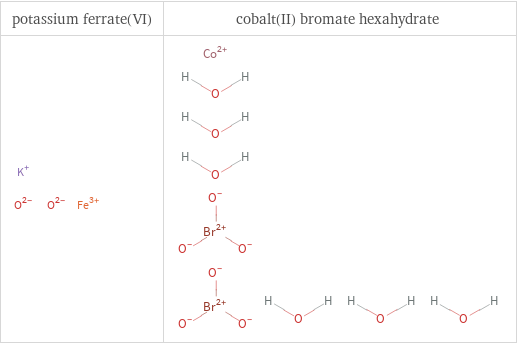
Structure diagrams
Basic properties
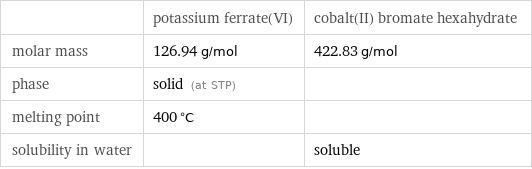
| potassium ferrate(VI) | cobalt(II) bromate hexahydrate molar mass | 126.94 g/mol | 422.83 g/mol phase | solid (at STP) | melting point | 400 °C | solubility in water | | soluble
Units
