Input interpretation

gaseous elements (at STP) | phase at STP
Summary
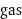
gas
Table
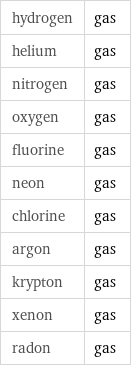
hydrogen | gas helium | gas nitrogen | gas oxygen | gas fluorine | gas neon | gas chlorine | gas argon | gas krypton | gas xenon | gas radon | gas
Thermodynamic properties
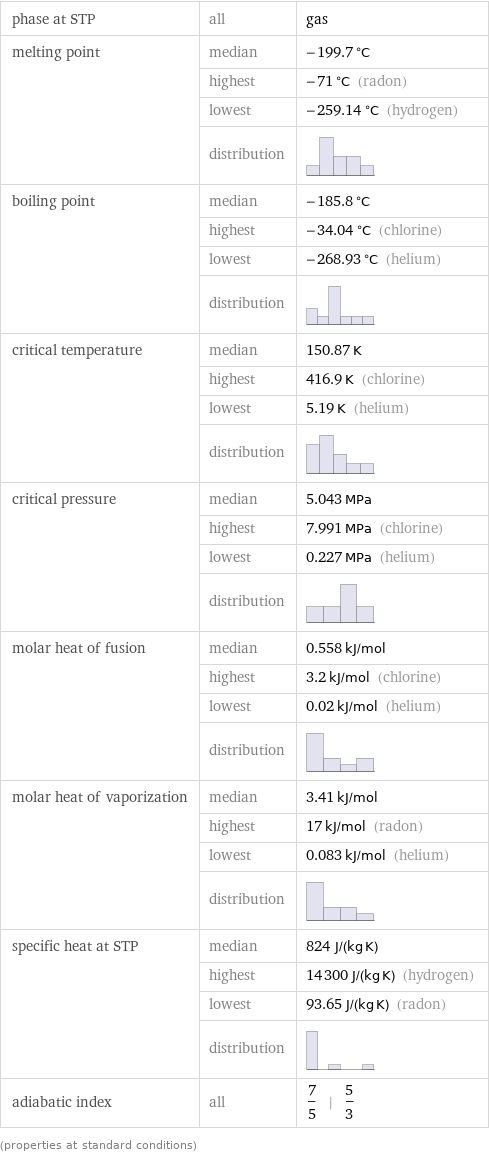
phase at STP | all | gas melting point | median | -199.7 °C | highest | -71 °C (radon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | -185.8 °C | highest | -34.04 °C (chlorine) | lowest | -268.93 °C (helium) | distribution | critical temperature | median | 150.87 K | highest | 416.9 K (chlorine) | lowest | 5.19 K (helium) | distribution | critical pressure | median | 5.043 MPa | highest | 7.991 MPa (chlorine) | lowest | 0.227 MPa (helium) | distribution | molar heat of fusion | median | 0.558 kJ/mol | highest | 3.2 kJ/mol (chlorine) | lowest | 0.02 kJ/mol (helium) | distribution | molar heat of vaporization | median | 3.41 kJ/mol | highest | 17 kJ/mol (radon) | lowest | 0.083 kJ/mol (helium) | distribution | specific heat at STP | median | 824 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 93.65 J/(kg K) (radon) | distribution | adiabatic index | all | 7/5 | 5/3 (properties at standard conditions)