Input interpretation
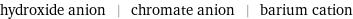
hydroxide anion | chromate anion | barium cation
Structure diagrams
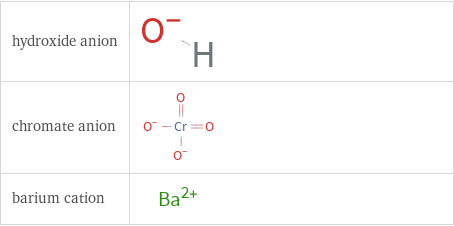
Structure diagrams
General properties
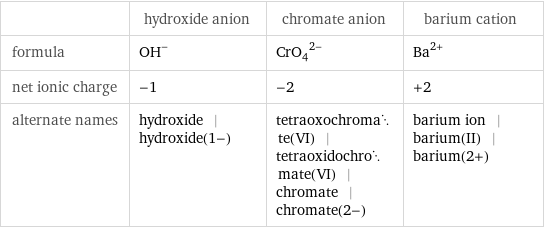
| hydroxide anion | chromate anion | barium cation formula | (OH)^- | (CrO_4)^(2-) | Ba^(2+) net ionic charge | -1 | -2 | +2 alternate names | hydroxide | hydroxide(1-) | tetraoxochromate(VI) | tetraoxidochromate(VI) | chromate | chromate(2-) | barium ion | barium(II) | barium(2+)
Ionic radii
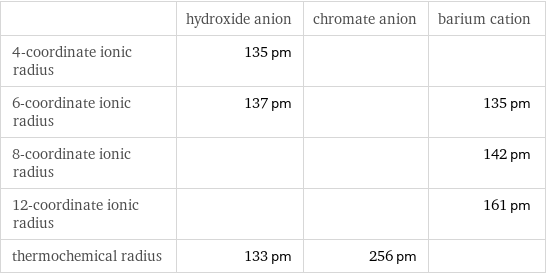
| hydroxide anion | chromate anion | barium cation 4-coordinate ionic radius | 135 pm | | 6-coordinate ionic radius | 137 pm | | 135 pm 8-coordinate ionic radius | | | 142 pm 12-coordinate ionic radius | | | 161 pm thermochemical radius | 133 pm | 256 pm |
Units

Other properties
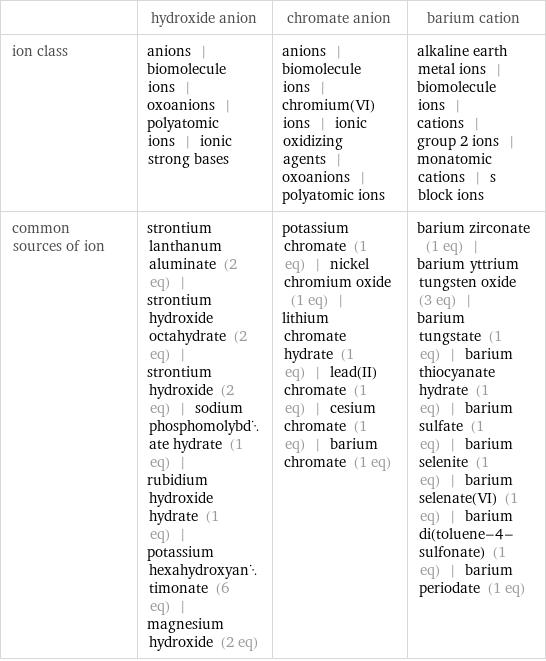
| hydroxide anion | chromate anion | barium cation ion class | anions | biomolecule ions | oxoanions | polyatomic ions | ionic strong bases | anions | biomolecule ions | chromium(VI) ions | ionic oxidizing agents | oxoanions | polyatomic ions | alkaline earth metal ions | biomolecule ions | cations | group 2 ions | monatomic cations | s block ions common sources of ion | strontium lanthanum aluminate (2 eq) | strontium hydroxide octahydrate (2 eq) | strontium hydroxide (2 eq) | sodium phosphomolybdate hydrate (1 eq) | rubidium hydroxide hydrate (1 eq) | potassium hexahydroxyantimonate (6 eq) | magnesium hydroxide (2 eq) | potassium chromate (1 eq) | nickel chromium oxide (1 eq) | lithium chromate hydrate (1 eq) | lead(II) chromate (1 eq) | cesium chromate (1 eq) | barium chromate (1 eq) | barium zirconate (1 eq) | barium yttrium tungsten oxide (3 eq) | barium tungstate (1 eq) | barium thiocyanate hydrate (1 eq) | barium sulfate (1 eq) | barium selenite (1 eq) | barium selenate(VI) (1 eq) | barium di(toluene-4-sulfonate) (1 eq) | barium periodate (1 eq)