Input interpretation
hypobromous acid | oxalic acid
Chemical names and formulas
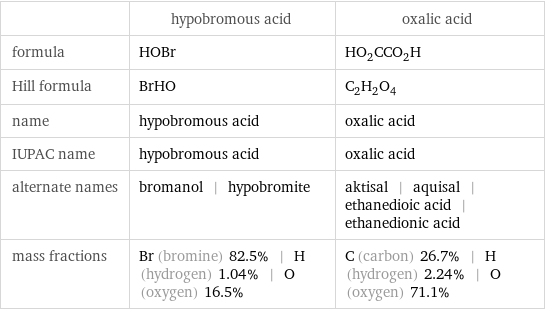
| hypobromous acid | oxalic acid formula | HOBr | HO_2CCO_2H Hill formula | BrHO | C_2H_2O_4 name | hypobromous acid | oxalic acid IUPAC name | hypobromous acid | oxalic acid alternate names | bromanol | hypobromite | aktisal | aquisal | ethanedioic acid | ethanedionic acid mass fractions | Br (bromine) 82.5% | H (hydrogen) 1.04% | O (oxygen) 16.5% | C (carbon) 26.7% | H (hydrogen) 2.24% | O (oxygen) 71.1%
Structure diagrams
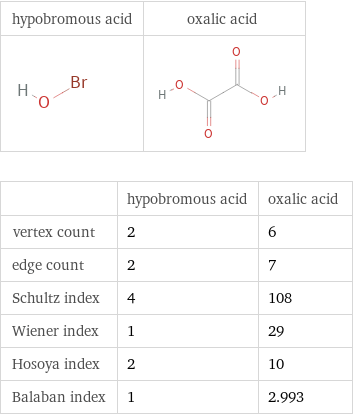
| hypobromous acid | oxalic acid vertex count | 2 | 6 edge count | 2 | 7 Schultz index | 4 | 108 Wiener index | 1 | 29 Hosoya index | 2 | 10 Balaban index | 1 | 2.993
3D structure
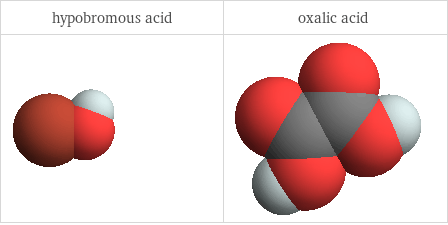
3D structure
Basic properties
| hypobromous acid | oxalic acid molar mass | 96.91 g/mol | 90.03 g/mol phase | | solid (at STP) melting point | | 189.5 °C boiling point | | 365 °C density | | 1.948 g/cm^3
Units

Hydrophobicity and permeability properties

| oxalic acid predicted LogP hydrophobicity | -0.5 experimental LogS | 0.38 predicted LogS | -0.14
Thermodynamic properties
| oxalic acid specific heat of vaporization | 0.746 kJ/g (kilojoules per gram)