Input interpretation

ammonium permanganate | elemental composition
Result
Find the elemental composition for ammonium permanganate in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: NH_4MnO_4 Use the chemical formula to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms, per molecule: | number of atoms H (hydrogen) | 4 Mn (manganese) | 1 N (nitrogen) | 1 O (oxygen) | 4 N_atoms = 4 + 1 + 1 + 4 = 10 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction H (hydrogen) | 4 | 4/10 Mn (manganese) | 1 | 1/10 N (nitrogen) | 1 | 1/10 O (oxygen) | 4 | 4/10 Check: 4/10 + 1/10 + 1/10 + 4/10 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent H (hydrogen) | 4 | 4/10 × 100% = 40.0% Mn (manganese) | 1 | 1/10 × 100% = 10.00% N (nitrogen) | 1 | 1/10 × 100% = 10.00% O (oxygen) | 4 | 4/10 × 100% = 40.0% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u H (hydrogen) | 4 | 40.0% | 1.008 Mn (manganese) | 1 | 10.00% | 54.938044 N (nitrogen) | 1 | 10.00% | 14.007 O (oxygen) | 4 | 40.0% | 15.999 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u H (hydrogen) | 4 | 40.0% | 1.008 | 4 × 1.008 = 4.032 Mn (manganese) | 1 | 10.00% | 54.938044 | 1 × 54.938044 = 54.938044 N (nitrogen) | 1 | 10.00% | 14.007 | 1 × 14.007 = 14.007 O (oxygen) | 4 | 40.0% | 15.999 | 4 × 15.999 = 63.996 m = 4.032 u + 54.938044 u + 14.007 u + 63.996 u = 136.973044 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction H (hydrogen) | 4 | 40.0% | 4.032/136.973044 Mn (manganese) | 1 | 10.00% | 54.938044/136.973044 N (nitrogen) | 1 | 10.00% | 14.007/136.973044 O (oxygen) | 4 | 40.0% | 63.996/136.973044 Check: 4.032/136.973044 + 54.938044/136.973044 + 14.007/136.973044 + 63.996/136.973044 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent H (hydrogen) | 4 | 40.0% | 4.032/136.973044 × 100% = 2.944% Mn (manganese) | 1 | 10.00% | 54.938044/136.973044 × 100% = 40.11% N (nitrogen) | 1 | 10.00% | 14.007/136.973044 × 100% = 10.23% O (oxygen) | 4 | 40.0% | 63.996/136.973044 × 100% = 46.72%
Mass fraction pie chart
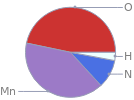
Mass fraction pie chart