Input interpretation
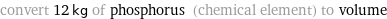
convert 12 kg of phosphorus (chemical element) to volume
Results
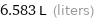
6.583 L (liters)
Possible intermediate steps
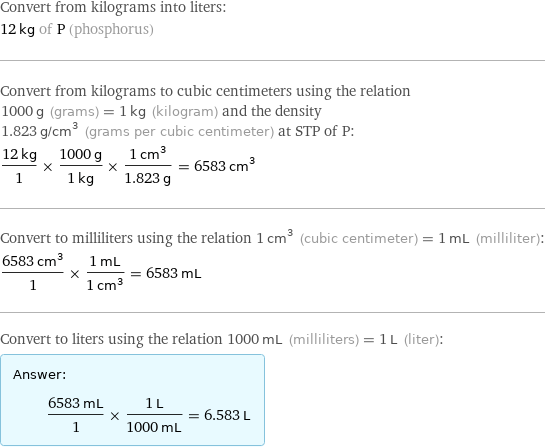
Convert from kilograms into liters: 12 kg of P (phosphorus) Convert from kilograms to cubic centimeters using the relation 1000 g (grams) = 1 kg (kilogram) and the density 1.823 g/cm^3 (grams per cubic centimeter) at STP of P: (12 kg)/1 × (1000 g)/(1 kg) × (1 cm^3)/(1.823 g) = 6583 cm^3 Convert to milliliters using the relation 1 cm^3 (cubic centimeter) = 1 mL (milliliter): (6583 cm^3)/1 × (1 mL)/(1 cm^3) = 6583 mL Convert to liters using the relation 1000 mL (milliliters) = 1 L (liter): Answer: | | (6583 mL)/1 × (1 L)/(1000 mL) = 6.583 L
Unit conversions
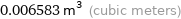
0.006583 m^3 (cubic meters)
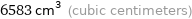
6583 cm^3 (cubic centimeters)
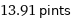
13.91 pints
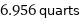
6.956 quarts
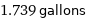
1.739 gallons
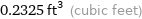
0.2325 ft^3 (cubic feet)
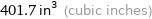
401.7 in^3 (cubic inches)
Comparisons as volume
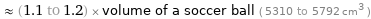
≈ (1.1 to 1.2) × volume of a soccer ball ( 5310 to 5792 cm^3 )
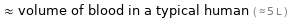
≈ volume of blood in a typical human ( ≈ 5 L )
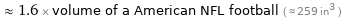
≈ 1.6 × volume of a American NFL football ( ≈ 259 in^3 )
Comparisons as volume of food or beverage
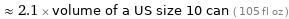
≈ 2.1 × volume of a US size 10 can ( 105 fl oz )
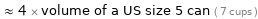
≈ 4 × volume of a US size 5 can ( 7 cups )

≈ 7 × volume of a US size 3 can ( 4 cups )
Interpretations
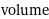
volume
volume of food or beverage
capacity
section modulus
Basic unit dimensions
![[length]^3](../image_source/82b0b22e35ebd3829ded0c8348f9645e.png)
[length]^3
Corresponding quantities
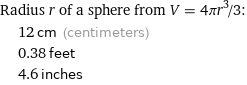
Radius r of a sphere from V = 4πr^3/3: | 12 cm (centimeters) | 0.38 feet | 4.6 inches
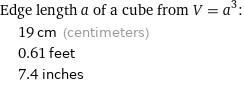
Edge length a of a cube from V = a^3: | 19 cm (centimeters) | 0.61 feet | 7.4 inches
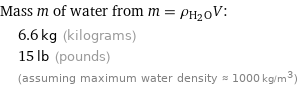
Mass m of water from m = ρ_(H_2O)V: | 6.6 kg (kilograms) | 15 lb (pounds) | (assuming maximum water density ≈ 1000 kg/m^3)

Molecule number N of water from N = ρ_(H_2O)V/m_(H_2O): | 2.2×10^26 molecules | (assuming maximum water density ≈ 1000 kg/m^3) | (assuming molecular mass of water ≈ 18.02 u)