Input interpretation

first order Arrhenius equation
Equation
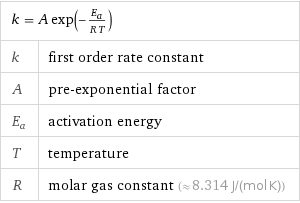
k = A exp(-E_a/(R T)) | k | first order rate constant A | pre-exponential factor E_a | activation energy T | temperature R | molar gas constant (≈ 8.314 J/(mol K))
Units

Input values

pre-exponential factor | 1×10^12 per second activation energy | 100 kJ/mol (kilojoules per mole) temperature | 298 K (kelvins)
Results
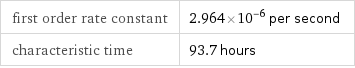
first order rate constant | 2.964×10^-6 per second characteristic time | 93.7 hours
Plot
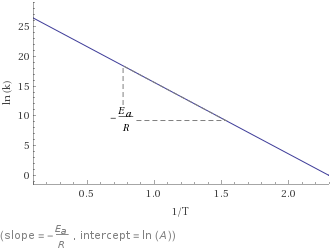
Plot