Input interpretation

solid compounds | vapor pressure
Summary

median | 133.3 Pa highest | 192500 Pa (potassium fluoride) lowest | 9×10^-27 Pa (aluminum) | (based on 13 values; 7 unavailable)
Entities with missing values
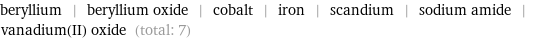
beryllium | beryllium oxide | cobalt | iron | scandium | sodium amide | vanadium(II) oxide (total: 7)
Distribution plots
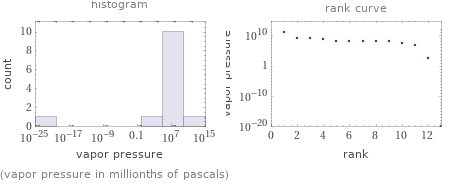
(vapor pressure in millionths of pascals)
Vapor pressure rankings
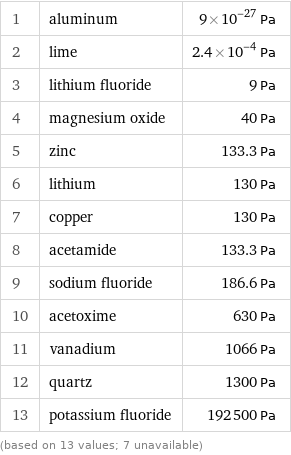
1 | aluminum | 9×10^-27 Pa 2 | lime | 2.4×10^-4 Pa 3 | lithium fluoride | 9 Pa 4 | magnesium oxide | 40 Pa 5 | zinc | 133.3 Pa 6 | lithium | 130 Pa 7 | copper | 130 Pa 8 | acetamide | 133.3 Pa 9 | sodium fluoride | 186.6 Pa 10 | acetoxime | 630 Pa 11 | vanadium | 1066 Pa 12 | quartz | 1300 Pa 13 | potassium fluoride | 192500 Pa (based on 13 values; 7 unavailable)
Unit conversions for median vapor pressure 133.3 Pa
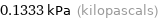
0.1333 kPa (kilopascals)

133.3 N/m^2 (newtons per square meter)
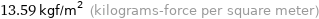
13.59 kgf/m^2 (kilograms-force per square meter)
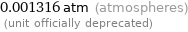
0.001316 atm (atmospheres) (unit officially deprecated)

0.001359 at (technical atmospheres) (unit officially deprecated)
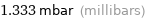
1.333 mbar (millibars)
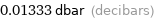
0.01333 dbar (decibars)
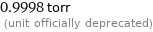
0.9998 torr (unit officially deprecated)
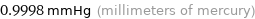
0.9998 mmHg (millimeters of mercury)
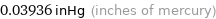
0.03936 inHg (inches of mercury)

1.359 cm WC (centimeters of water column)

13.59 mm WC (millimeters of water column)

0.01359 m WC (meters of water column)

0.5352 in WC (inches of water column (conventional))
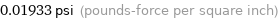
0.01933 psi (pounds-force per square inch)
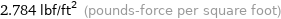
2.784 lbf/ft^2 (pounds-force per square foot)
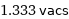
1.333 vacs
Comparisons for median vapor pressure 133.3 Pa
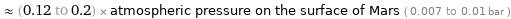
≈ (0.12 to 0.2) × atmospheric pressure on the surface of Mars ( 0.007 to 0.01 bar )
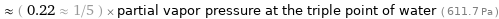
≈ ( 0.22 ≈ 1/5 ) × partial vapor pressure at the triple point of water ( 611.7 Pa )
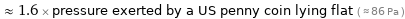
≈ 1.6 × pressure exerted by a US penny coin lying flat ( ≈ 86 Pa )
Corresponding quantities
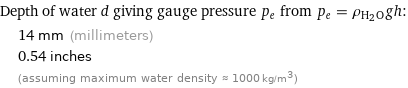
Depth of water d giving gauge pressure p_e from p_e = ρ_(H_2O)gh: | 14 mm (millimeters) | 0.54 inches | (assuming maximum water density ≈ 1000 kg/m^3)

Standard Atmosphere altitude: | 46 km (kilometers) | 29 miles | (based on 1976 US Standard Atmosphere)