Input interpretation

gadolinium (chemical element)
Periodic table location
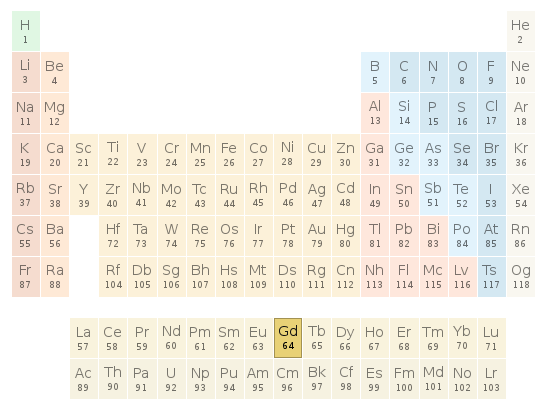
Periodic table location
Image

Image
Basic elemental properties
![atomic symbol | Gd atomic number | 64 short electronic configuration | [Xe]6s^24f^75d^1 Aufbau diagram | 5d 4f 6s block | f period | 6 atomic mass | 157.25 u (unified atomic mass units)](../image_source/3264f46d333ec1d443570bdf809f39ce.png)
atomic symbol | Gd atomic number | 64 short electronic configuration | [Xe]6s^24f^75d^1 Aufbau diagram | 5d 4f 6s block | f period | 6 atomic mass | 157.25 u (unified atomic mass units)
Thermodynamic properties
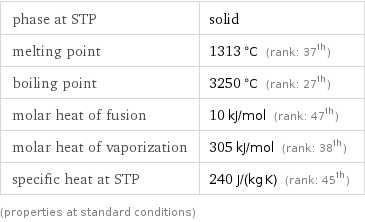
phase at STP | solid melting point | 1313 °C (rank: 37th) boiling point | 3250 °C (rank: 27th) molar heat of fusion | 10 kJ/mol (rank: 47th) molar heat of vaporization | 305 kJ/mol (rank: 38th) specific heat at STP | 240 J/(kg K) (rank: 45th) (properties at standard conditions)
Material properties
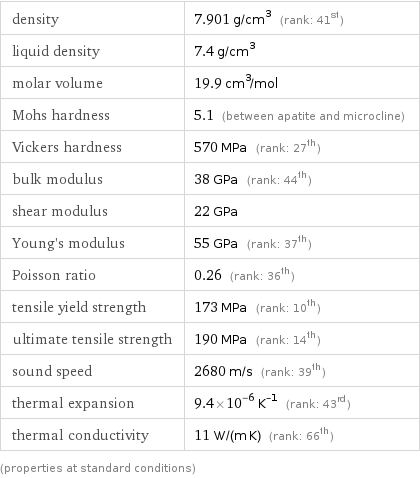
density | 7.901 g/cm^3 (rank: 41st) liquid density | 7.4 g/cm^3 molar volume | 19.9 cm^3/mol Mohs hardness | 5.1 (between apatite and microcline) Vickers hardness | 570 MPa (rank: 27th) bulk modulus | 38 GPa (rank: 44th) shear modulus | 22 GPa Young's modulus | 55 GPa (rank: 37th) Poisson ratio | 0.26 (rank: 36th) tensile yield strength | 173 MPa (rank: 10th) ultimate tensile strength | 190 MPa (rank: 14th) sound speed | 2680 m/s (rank: 39th) thermal expansion | 9.4×10^-6 K^(-1) (rank: 43rd) thermal conductivity | 11 W/(m K) (rank: 66th) (properties at standard conditions)
Electromagnetic properties
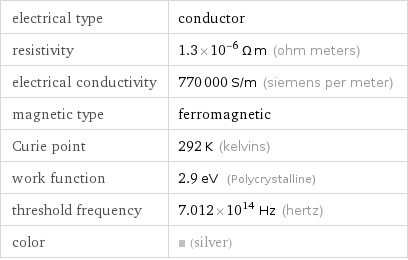
electrical type | conductor resistivity | 1.3×10^-6 Ω m (ohm meters) electrical conductivity | 770000 S/m (siemens per meter) magnetic type | ferromagnetic Curie point | 292 K (kelvins) work function | 2.9 eV (Polycrystalline) threshold frequency | 7.012×10^14 Hz (hertz) color | (silver)
Reactivity
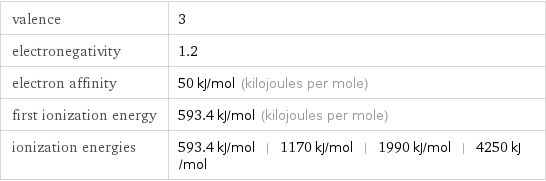
valence | 3 electronegativity | 1.2 electron affinity | 50 kJ/mol (kilojoules per mole) first ionization energy | 593.4 kJ/mol (kilojoules per mole) ionization energies | 593.4 kJ/mol | 1170 kJ/mol | 1990 kJ/mol | 4250 kJ/mol
Reactivity
Atomic properties

term symbol | ^9D_2 atomic radius | 180 pm covalent radius | 196 pm (electronic ground state properties)
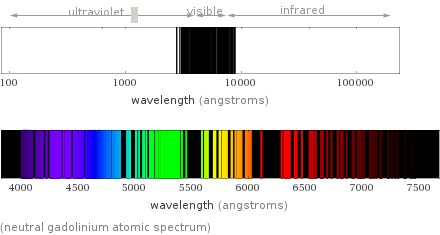
(neutral gadolinium atomic spectrum)
Abundances
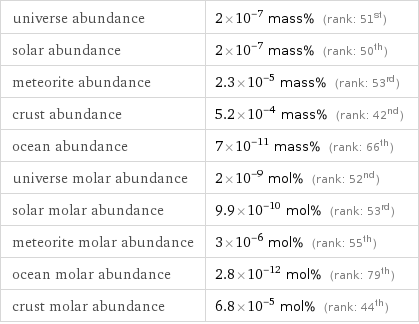
universe abundance | 2×10^-7 mass% (rank: 51st) solar abundance | 2×10^-7 mass% (rank: 50th) meteorite abundance | 2.3×10^-5 mass% (rank: 53rd) crust abundance | 5.2×10^-4 mass% (rank: 42nd) ocean abundance | 7×10^-11 mass% (rank: 66th) universe molar abundance | 2×10^-9 mol% (rank: 52nd) solar molar abundance | 9.9×10^-10 mol% (rank: 53rd) meteorite molar abundance | 3×10^-6 mol% (rank: 55th) ocean molar abundance | 2.8×10^-12 mol% (rank: 79th) crust molar abundance | 6.8×10^-5 mol% (rank: 44th)
Nuclear properties
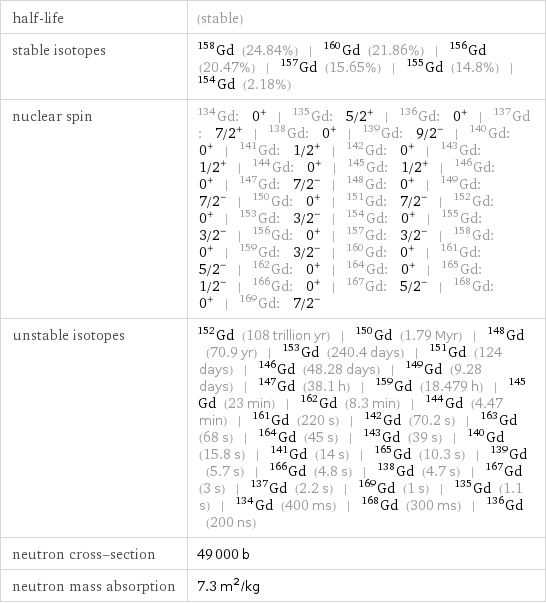
half-life | (stable) stable isotopes | Gd-158 (24.84%) | Gd-160 (21.86%) | Gd-156 (20.47%) | Gd-157 (15.65%) | Gd-155 (14.8%) | Gd-154 (2.18%) nuclear spin | Gd-134: 0^+ | Gd-135: 5/2^+ | Gd-136: 0^+ | Gd-137: 7/2^+ | Gd-138: 0^+ | Gd-139: 9/2^- | Gd-140: 0^+ | Gd-141: 1/2^+ | Gd-142: 0^+ | Gd-143: 1/2^+ | Gd-144: 0^+ | Gd-145: 1/2^+ | Gd-146: 0^+ | Gd-147: 7/2^- | Gd-148: 0^+ | Gd-149: 7/2^- | Gd-150: 0^+ | Gd-151: 7/2^- | Gd-152: 0^+ | Gd-153: 3/2^- | Gd-154: 0^+ | Gd-155: 3/2^- | Gd-156: 0^+ | Gd-157: 3/2^- | Gd-158: 0^+ | Gd-159: 3/2^- | Gd-160: 0^+ | Gd-161: 5/2^- | Gd-162: 0^+ | Gd-164: 0^+ | Gd-165: 1/2^- | Gd-166: 0^+ | Gd-167: 5/2^- | Gd-168: 0^+ | Gd-169: 7/2^- unstable isotopes | Gd-152 (108 trillion yr) | Gd-150 (1.79 Myr) | Gd-148 (70.9 yr) | Gd-153 (240.4 days) | Gd-151 (124 days) | Gd-146 (48.28 days) | Gd-149 (9.28 days) | Gd-147 (38.1 h) | Gd-159 (18.479 h) | Gd-145 (23 min) | Gd-162 (8.3 min) | Gd-144 (4.47 min) | Gd-161 (220 s) | Gd-142 (70.2 s) | Gd-163 (68 s) | Gd-164 (45 s) | Gd-143 (39 s) | Gd-140 (15.8 s) | Gd-141 (14 s) | Gd-165 (10.3 s) | Gd-139 (5.7 s) | Gd-166 (4.8 s) | Gd-138 (4.7 s) | Gd-167 (3 s) | Gd-137 (2.2 s) | Gd-169 (1 s) | Gd-135 (1.1 s) | Gd-134 (400 ms) | Gd-168 (300 ms) | Gd-136 (200 ns) neutron cross-section | 49000 b neutron mass absorption | 7.3 m^2/kg
Identifiers

CAS number | 7440-54-2 PubChem CID number | 23982