Input interpretation

benzene | UV cutoff wavelength
Result
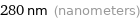
280 nm (nanometers)
Unit conversions
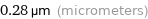
0.28 µm (micrometers)
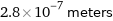
2.8×10^-7 meters
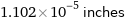
1.102×10^-5 inches
Comparison as wavelength
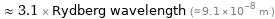
≈ 3.1 × Rydberg wavelength (≈ 9.1×10^-8 m )
Electromagnetic frequency range
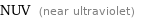
NUV (near ultraviolet)
Corresponding quantities
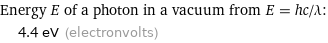
Energy E of a photon in a vacuum from E = hc/λ: | 4.4 eV (electronvolts)
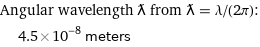
Angular wavelength ƛ from ƛ = λ/(2π): | 4.5×10^-8 meters
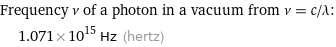
Frequency ν of a photon in a vacuum from ν = c/λ: | 1.071×10^15 Hz (hertz)
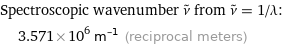
Spectroscopic wavenumber ν^~ from ν^~ = 1/λ: | 3.571×10^6 m^(-1) (reciprocal meters)
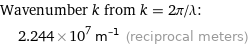
Wavenumber k from k = 2π/λ: | 2.244×10^7 m^(-1) (reciprocal meters)

Frequency ν of sound from ν = v/λ: | 1.2 GHz (gigahertz) | (assuming speed of sound ≈ 340.27 m/s)