Input interpretation

d-block elements | Néel point
Néel point rankings

1 | manganese | 100 K 2 | chromium | 393 K (based on 2 values; 38 unavailable)
Unit conversions for median Néel point 246 K

-27 °C (degrees Celsius)

-16 °F (degrees Fahrenheit)

444 °R (degrees Rankine)

-21 °Ré (degrees Réaumur)
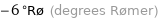
-6 °Rø (degrees Rømer)
Comparison for median Néel point 246 K

42 K below temperature at STP (standard temperature and pressure), using the convention of European and South American natural gas companies (15 °C)

27 K below temperature at STP (standard temperature and pressure), using the International Union of Pure and Applied Chemistry convention (0 °C)
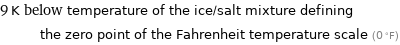
9 K below temperature of the ice/salt mixture defining the zero point of the Fahrenheit temperature scale (0 °F)
Corresponding quantities

Thermodynamic energy E from E = kT: | 21 meV (millielectronvolts)
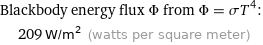
Blackbody energy flux Φ from Φ = σT^4: | 209 W/m^2 (watts per square meter)

Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 1.5×10^-27 lx (lux)
Thermodynamic properties
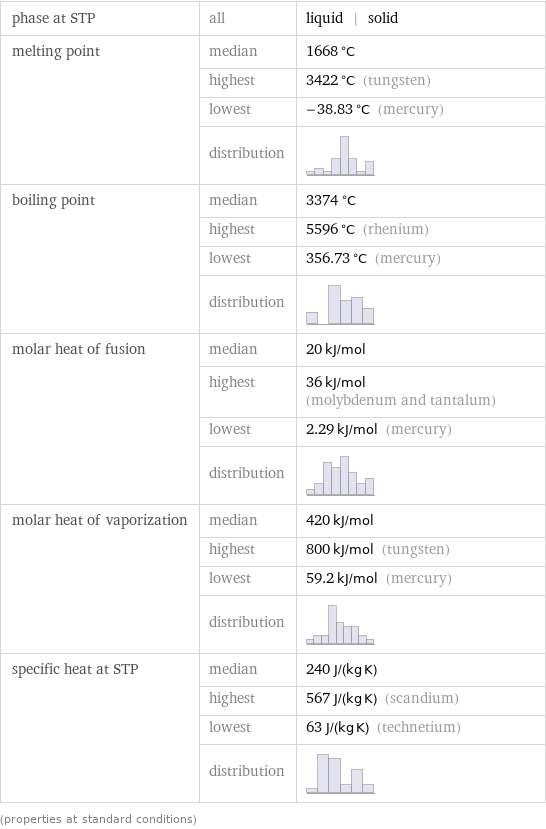
phase at STP | all | liquid | solid melting point | median | 1668 °C | highest | 3422 °C (tungsten) | lowest | -38.83 °C (mercury) | distribution | boiling point | median | 3374 °C | highest | 5596 °C (rhenium) | lowest | 356.73 °C (mercury) | distribution | molar heat of fusion | median | 20 kJ/mol | highest | 36 kJ/mol (molybdenum and tantalum) | lowest | 2.29 kJ/mol (mercury) | distribution | molar heat of vaporization | median | 420 kJ/mol | highest | 800 kJ/mol (tungsten) | lowest | 59.2 kJ/mol (mercury) | distribution | specific heat at STP | median | 240 J/(kg K) | highest | 567 J/(kg K) (scandium) | lowest | 63 J/(kg K) (technetium) | distribution | (properties at standard conditions)