Input interpretation
alkali metals | molar heat of vaporization
Summary
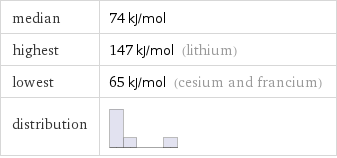
median | 74 kJ/mol highest | 147 kJ/mol (lithium) lowest | 65 kJ/mol (cesium and francium) distribution |
Units

Ranked values
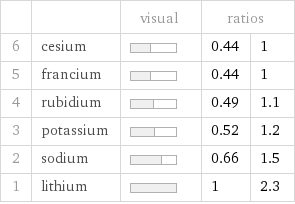
| | visual | ratios | 6 | cesium | | 0.44 | 1 5 | francium | | 0.44 | 1 4 | rubidium | | 0.49 | 1.1 3 | potassium | | 0.52 | 1.2 2 | sodium | | 0.66 | 1.5 1 | lithium | | 1 | 2.3
Molar heat of vaporization rankings
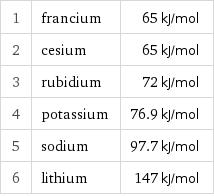
1 | francium | 65 kJ/mol 2 | cesium | 65 kJ/mol 3 | rubidium | 72 kJ/mol 4 | potassium | 76.9 kJ/mol 5 | sodium | 97.7 kJ/mol 6 | lithium | 147 kJ/mol
Unit conversions for median molar heat of vaporization 74 kJ/mol
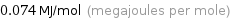
0.074 MJ/mol (megajoules per mole)
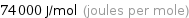
74000 J/mol (joules per mole)
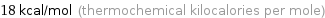
18 kcal/mol (thermochemical kilocalories per mole)
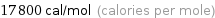
17800 cal/mol (calories per mole)
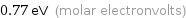
0.77 eV (molar electronvolts)