Input interpretation
chlorine (chemical element) | bromine (chemical element) | silicon (chemical element) | krypton (chemical element)
Periodic table location
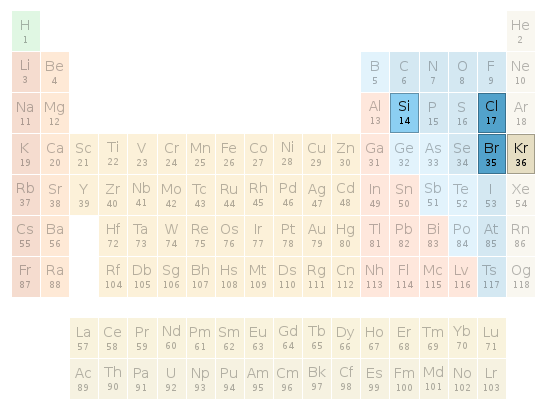
Periodic table location
Images
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration chlorine | Cl | 17 | [Ne]3s^23p^5 bromine | Br | 35 | [Ar]4s^23d^104p^5 silicon | Si | 14 | [Ne]3s^23p^2 krypton | Kr | 36 | [Ar]4s^23d^104p^6 | Aufbau diagram | block | group chlorine | 3p 3s | p | 17 bromine | 4p 3d 4s | p | 17 silicon | 3p 3s | p | 14 krypton | 4p 3d 4s | p | 18 | period | atomic mass | half-life chlorine | 3 | 35.45 u | (stable) bromine | 4 | 79.904 u | (stable) silicon | 3 | 28.085 u | (stable) krypton | 4 | 83.798 u | (stable)](../image_source/33431160931b359e2f824b5a6861c534.png)
| atomic symbol | atomic number | short electronic configuration chlorine | Cl | 17 | [Ne]3s^23p^5 bromine | Br | 35 | [Ar]4s^23d^104p^5 silicon | Si | 14 | [Ne]3s^23p^2 krypton | Kr | 36 | [Ar]4s^23d^104p^6 | Aufbau diagram | block | group chlorine | 3p 3s | p | 17 bromine | 4p 3d 4s | p | 17 silicon | 3p 3s | p | 14 krypton | 4p 3d 4s | p | 18 | period | atomic mass | half-life chlorine | 3 | 35.45 u | (stable) bromine | 4 | 79.904 u | (stable) silicon | 3 | 28.085 u | (stable) krypton | 4 | 83.798 u | (stable)
Thermodynamic properties
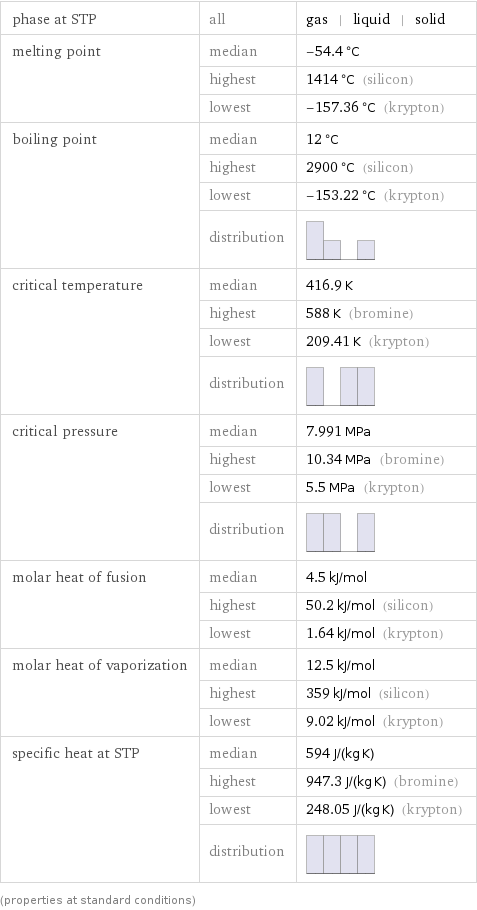
phase at STP | all | gas | liquid | solid melting point | median | -54.4 °C | highest | 1414 °C (silicon) | lowest | -157.36 °C (krypton) boiling point | median | 12 °C | highest | 2900 °C (silicon) | lowest | -153.22 °C (krypton) | distribution | critical temperature | median | 416.9 K | highest | 588 K (bromine) | lowest | 209.41 K (krypton) | distribution | critical pressure | median | 7.991 MPa | highest | 10.34 MPa (bromine) | lowest | 5.5 MPa (krypton) | distribution | molar heat of fusion | median | 4.5 kJ/mol | highest | 50.2 kJ/mol (silicon) | lowest | 1.64 kJ/mol (krypton) molar heat of vaporization | median | 12.5 kJ/mol | highest | 359 kJ/mol (silicon) | lowest | 9.02 kJ/mol (krypton) specific heat at STP | median | 594 J/(kg K) | highest | 947.3 J/(kg K) (bromine) | lowest | 248.05 J/(kg K) (krypton) | distribution | (properties at standard conditions)
Material properties
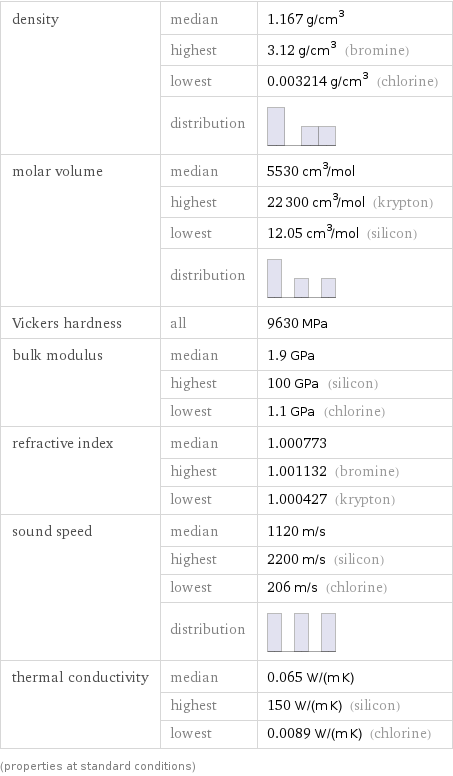
density | median | 1.167 g/cm^3 | highest | 3.12 g/cm^3 (bromine) | lowest | 0.003214 g/cm^3 (chlorine) | distribution | molar volume | median | 5530 cm^3/mol | highest | 22300 cm^3/mol (krypton) | lowest | 12.05 cm^3/mol (silicon) | distribution | Vickers hardness | all | 9630 MPa bulk modulus | median | 1.9 GPa | highest | 100 GPa (silicon) | lowest | 1.1 GPa (chlorine) refractive index | median | 1.000773 | highest | 1.001132 (bromine) | lowest | 1.000427 (krypton) sound speed | median | 1120 m/s | highest | 2200 m/s (silicon) | lowest | 206 m/s (chlorine) | distribution | thermal conductivity | median | 0.065 W/(m K) | highest | 150 W/(m K) (silicon) | lowest | 0.0089 W/(m K) (chlorine) (properties at standard conditions)
Electromagnetic properties
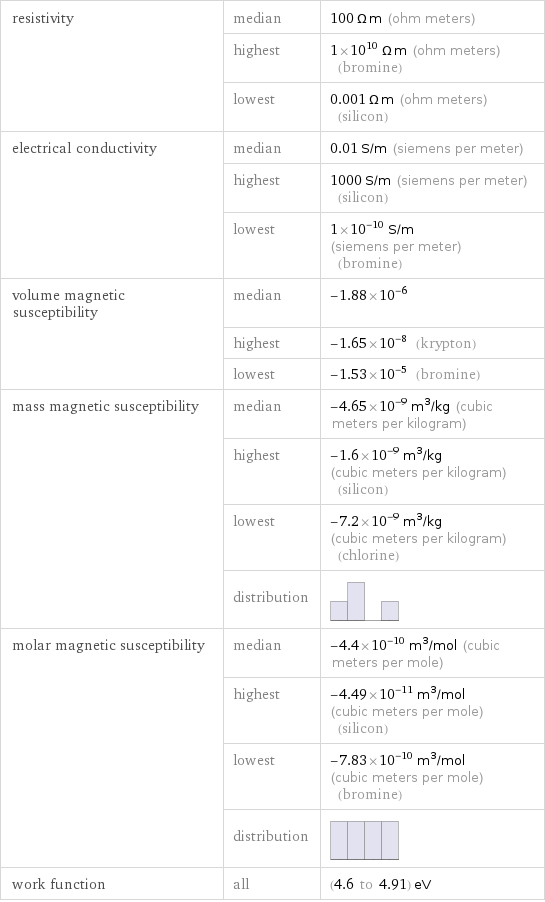
resistivity | median | 100 Ω m (ohm meters) | highest | 1×10^10 Ω m (ohm meters) (bromine) | lowest | 0.001 Ω m (ohm meters) (silicon) electrical conductivity | median | 0.01 S/m (siemens per meter) | highest | 1000 S/m (siemens per meter) (silicon) | lowest | 1×10^-10 S/m (siemens per meter) (bromine) volume magnetic susceptibility | median | -1.88×10^-6 | highest | -1.65×10^-8 (krypton) | lowest | -1.53×10^-5 (bromine) mass magnetic susceptibility | median | -4.65×10^-9 m^3/kg (cubic meters per kilogram) | highest | -1.6×10^-9 m^3/kg (cubic meters per kilogram) (silicon) | lowest | -7.2×10^-9 m^3/kg (cubic meters per kilogram) (chlorine) | distribution | molar magnetic susceptibility | median | -4.4×10^-10 m^3/mol (cubic meters per mole) | highest | -4.49×10^-11 m^3/mol (cubic meters per mole) (silicon) | lowest | -7.83×10^-10 m^3/mol (cubic meters per mole) (bromine) | distribution | work function | all | (4.6 to 4.91) eV
Reactivity
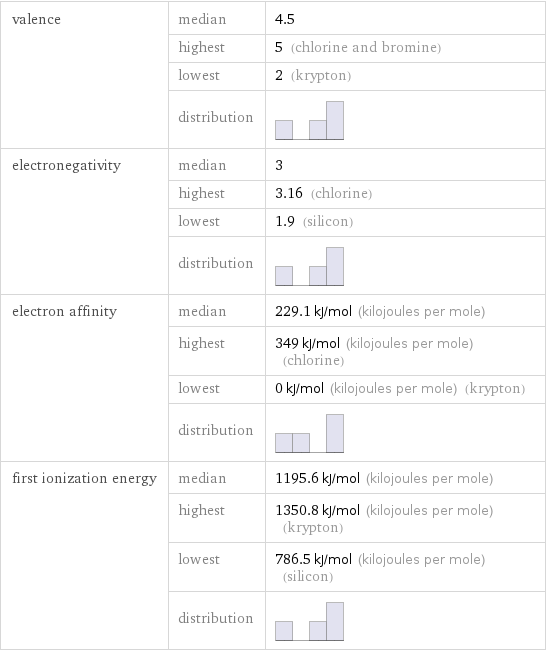
valence | median | 4.5 | highest | 5 (chlorine and bromine) | lowest | 2 (krypton) | distribution | electronegativity | median | 3 | highest | 3.16 (chlorine) | lowest | 1.9 (silicon) | distribution | electron affinity | median | 229.1 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 0 kJ/mol (kilojoules per mole) (krypton) | distribution | first ionization energy | median | 1195.6 kJ/mol (kilojoules per mole) | highest | 1350.8 kJ/mol (kilojoules per mole) (krypton) | lowest | 786.5 kJ/mol (kilojoules per mole) (silicon) | distribution |
Atomic properties
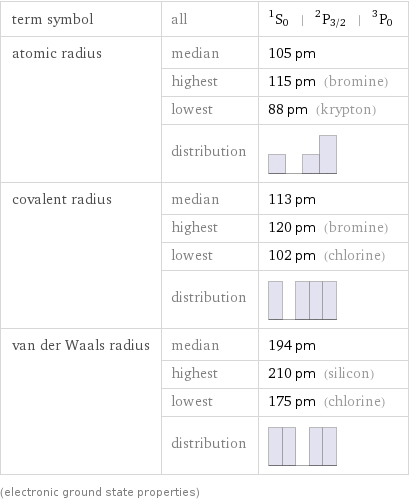
term symbol | all | ^1S_0 | ^2P_(3/2) | ^3P_0 atomic radius | median | 105 pm | highest | 115 pm (bromine) | lowest | 88 pm (krypton) | distribution | covalent radius | median | 113 pm | highest | 120 pm (bromine) | lowest | 102 pm (chlorine) | distribution | van der Waals radius | median | 194 pm | highest | 210 pm (silicon) | lowest | 175 pm (chlorine) | distribution | (electronic ground state properties)