Input interpretation
hydronium cation
Lewis structure
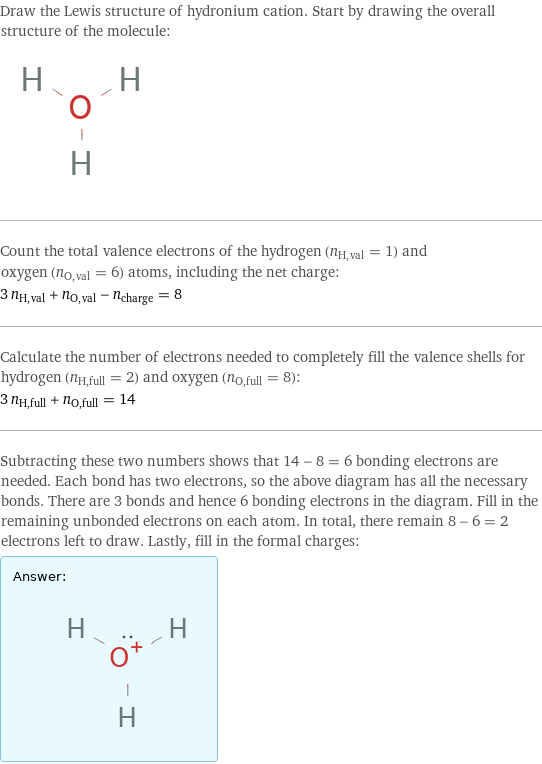
Draw the Lewis structure of hydronium cation. Start by drawing the overall structure of the molecule: Count the total valence electrons of the hydrogen (n_H, val = 1) and oxygen (n_O, val = 6) atoms, including the net charge: 3 n_H, val + n_O, val - n_charge = 8 Calculate the number of electrons needed to completely fill the valence shells for hydrogen (n_H, full = 2) and oxygen (n_O, full = 8): 3 n_H, full + n_O, full = 14 Subtracting these two numbers shows that 14 - 8 = 6 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 3 bonds and hence 6 bonding electrons in the diagram. Fill in the remaining unbonded electrons on each atom. In total, there remain 8 - 6 = 2 electrons left to draw. Lastly, fill in the formal charges: Answer: | |
General properties
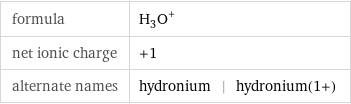
formula | (H_3O)^+ net ionic charge | +1 alternate names | hydronium | hydronium(1+)
Other properties
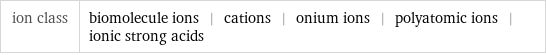
ion class | biomolecule ions | cations | onium ions | polyatomic ions | ionic strong acids