Input interpretation
lithium aluminum hydride
Chemical names and formulas
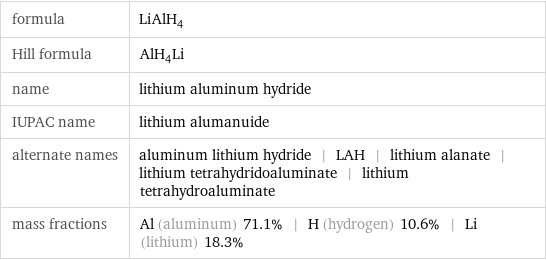
formula | LiAlH_4 Hill formula | AlH_4Li name | lithium aluminum hydride IUPAC name | lithium alumanuide alternate names | aluminum lithium hydride | LAH | lithium alanate | lithium tetrahydridoaluminate | lithium tetrahydroaluminate mass fractions | Al (aluminum) 71.1% | H (hydrogen) 10.6% | Li (lithium) 18.3%
Structure diagram
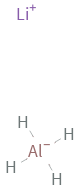
Structure diagram
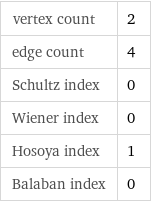
vertex count | 2 edge count | 4 Schultz index | 0 Wiener index | 0 Hosoya index | 1 Balaban index | 0
Basic properties
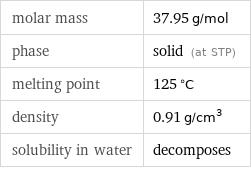
molar mass | 37.95 g/mol phase | solid (at STP) melting point | 125 °C density | 0.91 g/cm^3 solubility in water | decomposes
Units

Solid properties (at STP)

density | 0.91 g/cm^3
Units

Thermodynamic properties
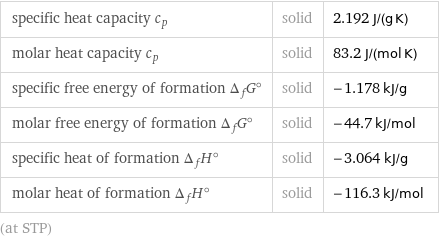
specific heat capacity c_p | solid | 2.192 J/(g K) molar heat capacity c_p | solid | 83.2 J/(mol K) specific free energy of formation Δ_fG° | solid | -1.178 kJ/g molar free energy of formation Δ_fG° | solid | -44.7 kJ/mol specific heat of formation Δ_fH° | solid | -3.064 kJ/g molar heat of formation Δ_fH° | solid | -116.3 kJ/mol (at STP)
Chemical identifiers
![CAS number | 16853-85-3 PubChem CID number | 28112 PubChem SID number | 8146274 SMILES identifier | [Li+].[AlH4-] InChI identifier | InChI=1/Al.Li.4H/q-1;+1;;;;/rAlH4.Li/h1H4;/q-1;+1 RTECS number | BD0100000 MDL number | MFCD00011075](../image_source/d9933f5c82fc12ffa5f7298558c3bca4.png)
CAS number | 16853-85-3 PubChem CID number | 28112 PubChem SID number | 8146274 SMILES identifier | [Li+].[AlH4-] InChI identifier | InChI=1/Al.Li.4H/q-1;+1;;;;/rAlH4.Li/h1H4;/q-1;+1 RTECS number | BD0100000 MDL number | MFCD00011075
NFPA label

NFPA label
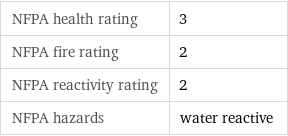
NFPA health rating | 3 NFPA fire rating | 2 NFPA reactivity rating | 2 NFPA hazards | water reactive
Safety properties

flash point | 125 °C

DOT hazard class | 4.3 DOT numbers | 1411
Toxicity properties

odor | odorless

RTECS classes | other