Input interpretation

inorganic salts | freezing point
Summary
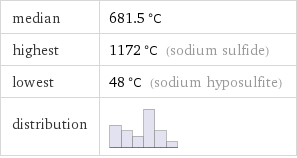
median | 681.5 °C highest | 1172 °C (sodium sulfide) lowest | 48 °C (sodium hyposulfite) distribution |
Distribution plots
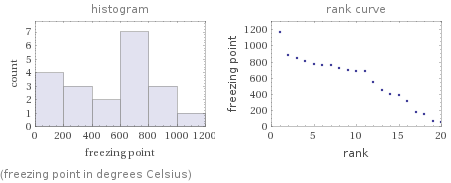
(freezing point in degrees Celsius)
Freezing point rankings
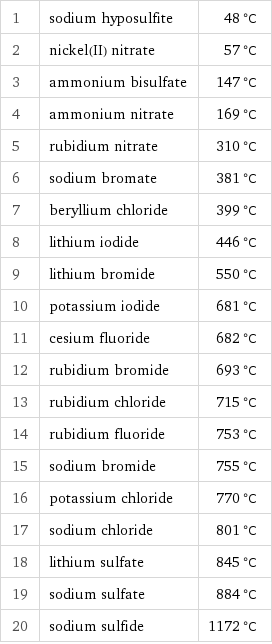
1 | sodium hyposulfite | 48 °C 2 | nickel(II) nitrate | 57 °C 3 | ammonium bisulfate | 147 °C 4 | ammonium nitrate | 169 °C 5 | rubidium nitrate | 310 °C 6 | sodium bromate | 381 °C 7 | beryllium chloride | 399 °C 8 | lithium iodide | 446 °C 9 | lithium bromide | 550 °C 10 | potassium iodide | 681 °C 11 | cesium fluoride | 682 °C 12 | rubidium bromide | 693 °C 13 | rubidium chloride | 715 °C 14 | rubidium fluoride | 753 °C 15 | sodium bromide | 755 °C 16 | potassium chloride | 770 °C 17 | sodium chloride | 801 °C 18 | lithium sulfate | 845 °C 19 | sodium sulfate | 884 °C 20 | sodium sulfide | 1172 °C