Input interpretation

ionic diatomics | molar heat of formation Δ_fH°
Table
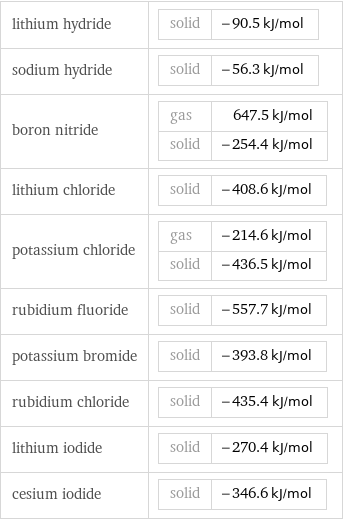
lithium hydride | solid | -90.5 kJ/mol sodium hydride | solid | -56.3 kJ/mol boron nitride | gas | 647.5 kJ/mol solid | -254.4 kJ/mol lithium chloride | solid | -408.6 kJ/mol potassium chloride | gas | -214.6 kJ/mol solid | -436.5 kJ/mol rubidium fluoride | solid | -557.7 kJ/mol potassium bromide | solid | -393.8 kJ/mol rubidium chloride | solid | -435.4 kJ/mol lithium iodide | solid | -270.4 kJ/mol cesium iodide | solid | -346.6 kJ/mol
Units
